- Department of Radiology, Division of Neuroradiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, United States.
- Department of Neurosurgery, University of Texas-Southwestern Medical Center, Dallas, Texas, United States.
- Department of Medicine Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, United States.
- Department of Neurosurgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, United States.
Correspondence Address:
Suyash Mohan, Department of Radiology, Division of Neuroradiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, United States.
DOI:10.25259/SNI_353_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Suyash Mohan1, Sumei Wang1, Sanjeev Chawla1, Kalil Abdullah2, Arati Desai3, Eileen Maloney4, Steven Brem4. Multiparametric MRI assessment of response to convection-enhanced intratumoral delivery of MDNA55, an interleukin-4 receptor targeted immunotherapy, for recurrent glioblastoma. 06-Jul-2021;12:337
How to cite this URL: Suyash Mohan1, Sumei Wang1, Sanjeev Chawla1, Kalil Abdullah2, Arati Desai3, Eileen Maloney4, Steven Brem4. Multiparametric MRI assessment of response to convection-enhanced intratumoral delivery of MDNA55, an interleukin-4 receptor targeted immunotherapy, for recurrent glioblastoma. 06-Jul-2021;12:337. Available from: https://surgicalneurologyint.com/surgicalint-articles/10941/
Abstract
Background: Glioblastoma (GBM) is the most common malignant brain tumor and carries a dismal prognosis. Attempts to develop biologically targeted therapies are challenging as the blood–brain barrier can limit drugs from reaching their target when administered through conventional (intravenous or oral) routes. Furthermore, systemic toxicity of drugs often limits their therapeutic potential. To circumvent these problems, convection-enhanced delivery (CED) provides direct, targeted, intralesional therapy with a secondary objective to alter the tumor microenvironment from an immunologically “cold” (nonresponsive) to an “inflamed” (immunoresponsive) tumor.
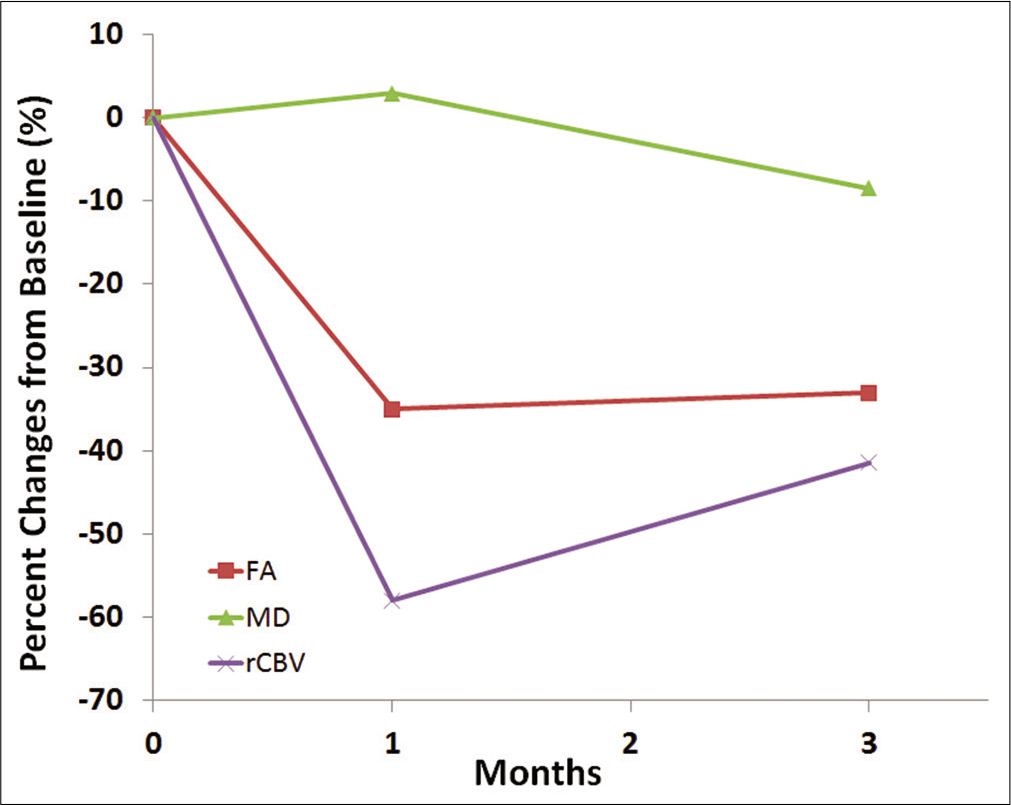
Case Description: We report a patient with right occipital recurrent GBM harboring poor prognostic genotypes who was treated with MRI-guided CED of a fusion protein MDNA55 (a targeted toxin directed toward the interleukin-4 receptor). The patient underwent serial anatomical, diffusion, and perfusion MRI scans before initiation of targeted therapy and at 1, 3-month posttherapy. Increased mean diffusivity along with decreased fractional anisotropy and maximum relative cerebral blood volume was noted at follow-up periods relative to baseline.
Conclusion: Our findings suggest that diffusion and perfusion MRI techniques may be useful in evaluating early response to CED of MDNA55 in recurrent GBM patients.
Keywords: Diffusion tensor imaging dynamic susceptibility contrast, Multiparametric MRI, Recurrent glioblastoma, Response assessment
INTRODUCTION
Glioblastoma (GBM) is the most common aggressive primary malignant brain tumor in adults with a miserable prognosis.[
At present,[
CLINICAL DESCRIPTION
A 57-year-old female with the right occipital recurrent GBM (IDH-1 wild-type, MGMT promoter methylated, with EGFR amplification) was treated with MRI-guided convection-enhanced intratumoral delivery of MDNA55. She underwent initial resection 32 months before this presentation followed by standard-of-care chemoradiation therapy. Her follow-up MRI demonstrated increasing size of enhancing lesion along the anteroinferior aspect of the right occipital resection cavity with elevated relative cerebral blood volume (rCBV), consistent with progressive recurrent malignant glial neoplasm. At this time, her presenting symptoms included memory deficits, confusion, persistent fatigue, and worsening gait instability.
Placement of catheters and infusion of MDNA55
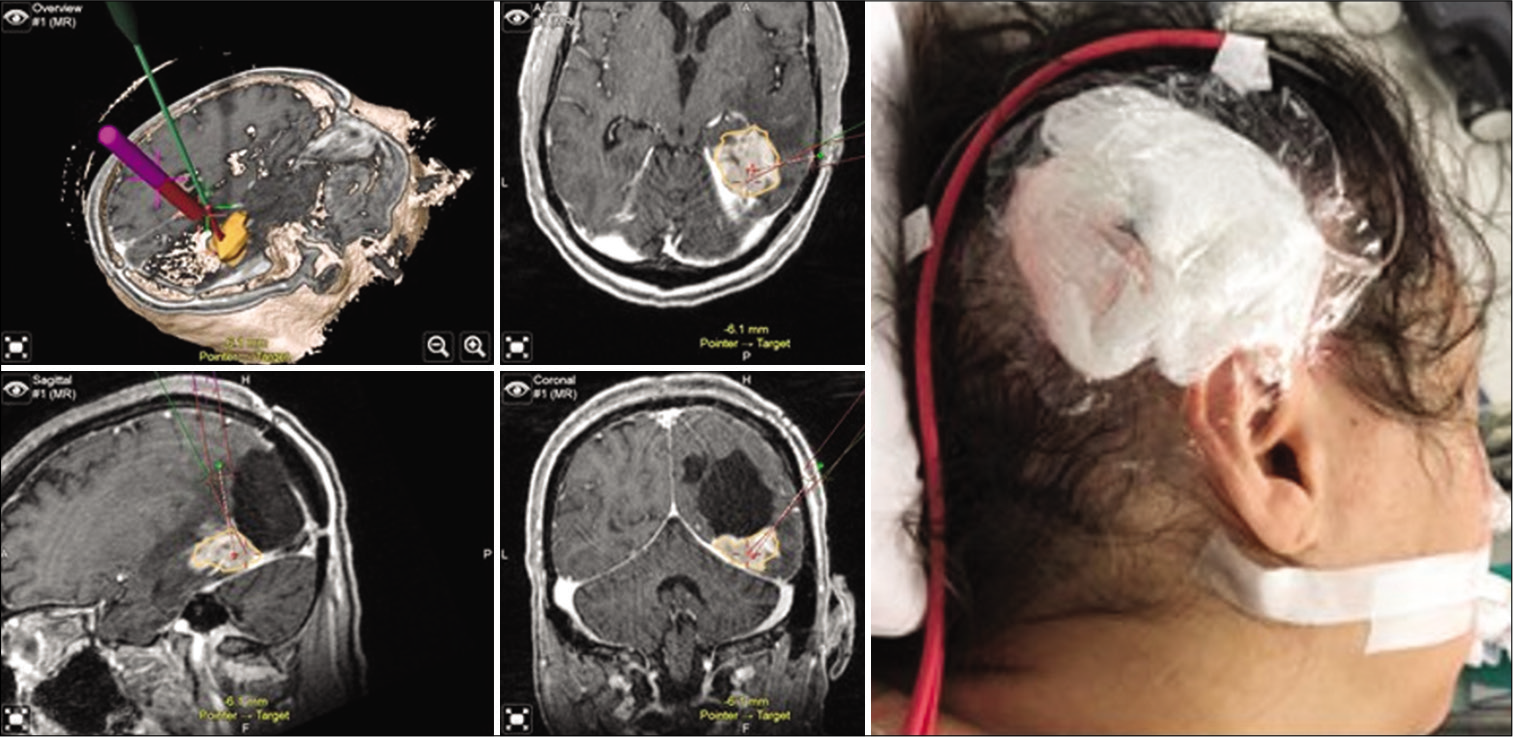
CED catheters were precisely placed under stereotactic guidance after importing the neuronavigation sequences to Brainlab Curve-100 workstation. Two entry points were planned for the minimally invasive trajectory using the “Overview” view to develop a 3D model of the target recurrent tumor and surrounding neural and vascular structures. The catheters were secured in a 14 French Foley catheter (red rubber tubing), stab wounds were then closed with 3-0 Nylon (Neurolon) sutures and the patient was transferred, intubated to the MRI suite [
Figure 1:
Intraoperative neuronavigation (Brainlab®, Munich, Germany) demonstrating lesion mapping and trajectory planning through topographic, axial, sagittal, and coronal views (clockwise, left panel). Postoperative wound dressing and secured drug delivery catheter, reflecting the right occipital-parietal entry point (right panel).
Imaging response assessment
The patient underwent serial MRI scans including a baseline and 1, 3-month follow-up MRI on a 3T scanner using a 12-channel, phased array head coil. The MRI protocol included anatomical images, diffusion tensor imaging (DTI), and dynamic susceptibility contrast perfusion-weighted imaging (DSC-PWI) using parameters as described previously.[
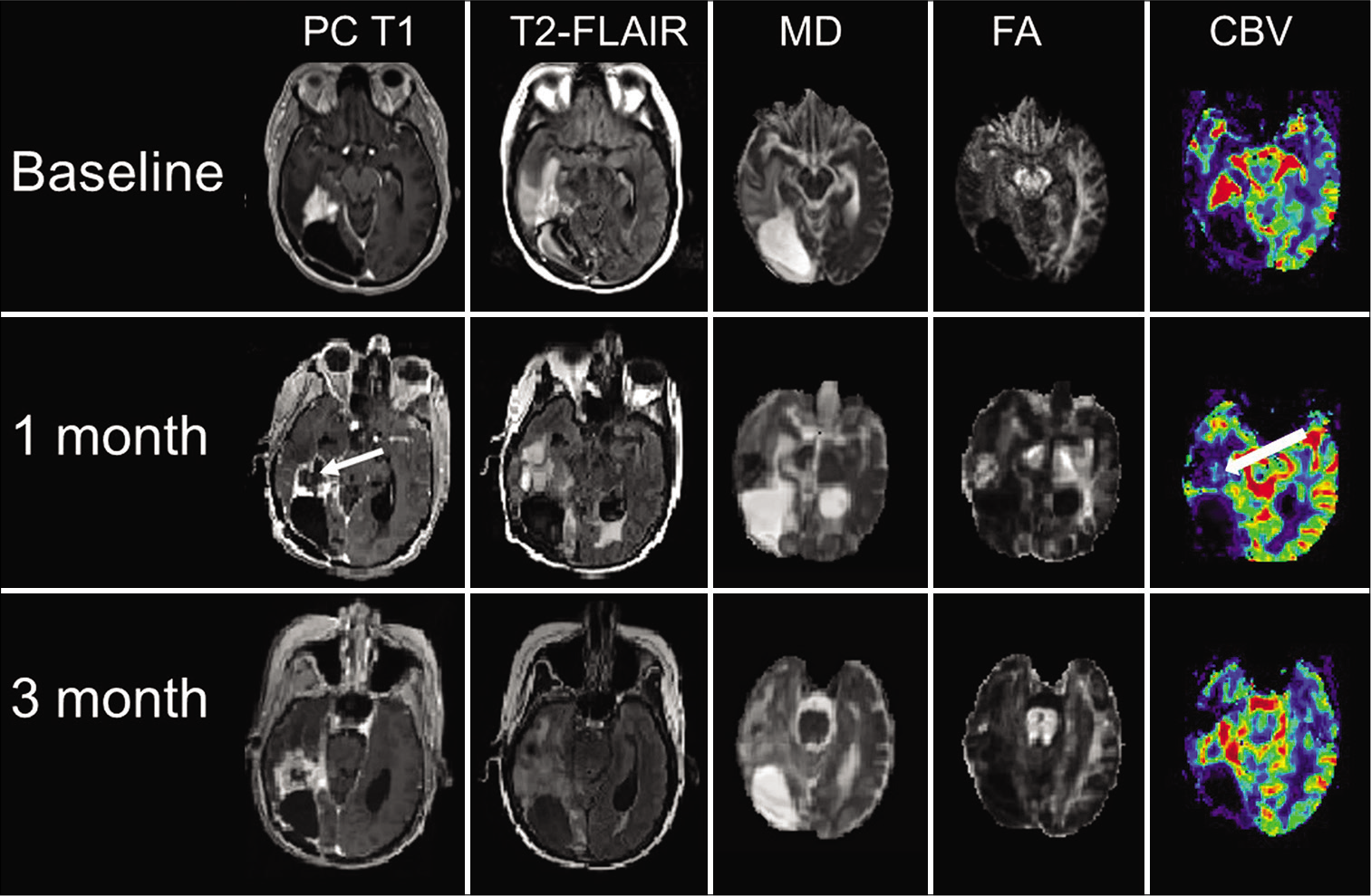
Figure 3:
Axial coregistered postcontrast T1-weighted image (PC T1), T2-FLAIR, and corresponding mean diffusivity (MD), fractional anisotropy (FA), and cerebral blood volume (CBV) maps are shown at baseline, 1-month, and at 3-month follow-up periods. There was a striking reduction of contrast enhancement, and CBV (white arrows), more apparent at 1-month follow-up compared to the baseline, with increased MD and decreased FA.
Clinical follow-up
She did well immediately after the infusion, was neurologically intact, and discharged to a rehabilitation facility on day 2 after the procedure. She was readmitted after approximately 1½ months with declining Karnofsky performance score (KPS of 50), persistent nausea and vomiting, unsteadiness of gait, and somnolence, and was found to have hydrocephalus for which a ventriculoperitoneal shunt was placed. She eventually experienced tumor progression and was not considered a candidate for additional treatment, and hence, was transferred to hospice for palliative care.
DISCUSSION
We demonstrated imaging findings suggestive of a positive early response to convection-enhanced intratumoral delivery of MDNA55, an IL-4 receptor targeted immunotherapy, in a patient with recurrent GBM. Reduced tumor volume, increased MD, decreased FA, and markedly reduced rCBV were observed from contrast enhancing regions of neoplasm. As tissue immunophenotyping was not performed, it is speculative whether the favorable local response was due to a direct pharmacological effect, or an induced immunomodulatory effect, or a combination of these.
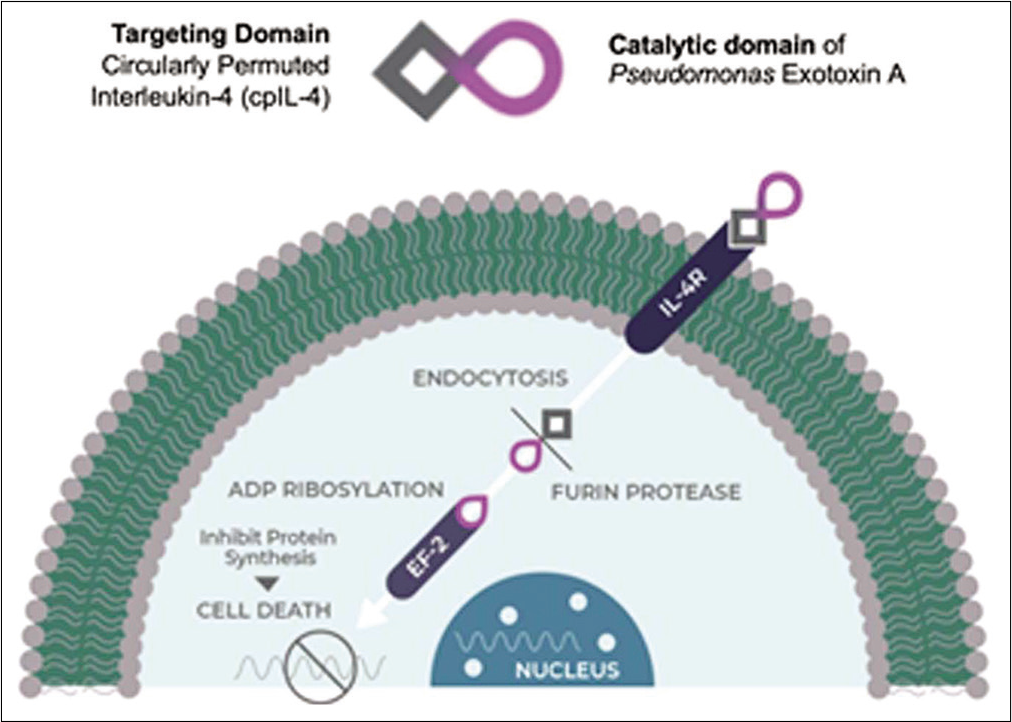
The IL-4R is highly expressed in multiple tumor types including 76% of GBMs, spurring tumor growth.[
Conventional MRI is limited in evaluating treatment response in GBM patients, due to lack of specificity, especially in the setting of immunotherapy.[
Neovascularization (formation of new blood vessels) is a common feature of GBMs that account for high tumor perfusion as seen on DSC-PWI-derived CBV maps.[
Despite showing promising findings, clinical utility of CBV maps sometimes may be constrained by limitations that include susceptibility artifacts caused by microhemorrhages present within the tumor bed.[
We believe that multiparametric analysis combining the unique strengths of DTI and DSC-MRI techniques, as performed in the present case, could contribute to a more comprehensive assessment of treatment response in these patients.
CONCLUSION
Advanced MRI techniques could be a useful adjunct in assessing early response to CED of MDNA55 in recurrent GBM patients. However, this promising finding warrants further validation in future, larger clinical trials.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal A, Kumar S, Narang J, Schultz L, Mikkelsen T, Wang S. Morphologic MRI features, diffusion tensor imaging and radiation dosimetric analysis to differentiate pseudo-progression from early tumor progression. J Neurooncol. 2013. 112: 413-20
2. Aquino D, Di Stefano AL, Scotti A, Cuppini C, Anghileri E, Finocchiaro G. Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to bevacizumab and irinotecan. PLoS One. 2014. 9: e90535
3. Beppu T, Inoue T, Shibata Y, Yamada N, Kurose A, Ogasawara K. Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neur. 2005. 63: 56-61
4. Bisdas S, Smrdel U, Bajrovic FF, Surlan-Popovic K. Assessment of progression-free-survival in glioblastomas by intratreatment dynamic contrast-enhanced MRI. Clin Neuroradiol. 2016. 26: 39-45
5. Chawla S, Shehu V, Gupta PK, Nath K, Poptani H. Physiological imaging methods for evaluating response to immunotherapies in glioblastomas. Int J Mol Sci. 2021. 22: 3867
6. Chawla S, Wang S, Kim S, Sheriff S, Lee P, Rengan R. Radiation injury to the normal brain measured by 3D-echo-planar spectroscopic imaging and diffusion tensor imaging: initial experience. J. Neuroimaging. 2015. 25: 97-104
7. Chawla S, Wang S, Mohan S, Nasrallah M, Verma G, Brem S. Differentiation of brain infection from necrotic glioblastoma using combined analysis of diffusion and perfusion MRI. J Magn Reson Imaging. 2019. 49: 184-94
8. Floeth FW, Wittsack HJ, Engelbrecht V, Weber F. Comparative follow-up of enhancement phenomena with MRI and Proton MR Spectroscopic Imaging after intralesional immunotherapy in glioblastoma--report of two exceptional cases. Zentralbl Neurochir. 2002. 63: 23-8
9. Han J, Puri RK. Analysis of the cancer genome Atlas (TCGA) database identifies an inverse relationship between interleukin-13 receptor α1 and α2 gene expression and poor prognosis and drug resistance in subjects with glioblastoma multiforme. J Neurooncol. 2018. 136: 463-74
10. Huber T, Bette S, Wiestler B, Gempt J, Gerhardt J, Delbridge C. Fractional anisotropy correlates with overall survival in glioblastoma. World Neurosurg. 2016. 95: 525-34.e1
11. Jackson EF, Barboriak DP, Bidaut LM, Meyer CR. Magnetic resonance assessment of response to therapy: Tumor change measurement, truth data and error sources. Transl Oncol. 2009. 2: 211-5
12. Jahng GH, Li KL, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: A comprehensive update on principles and techniques. Korean J Radiol. 2014. 15: 554-77
13. Jeon JY, Kovanlikaya I, Boockvar JA, Mao X, Shin B, Burkhardt JK. Metabolic response of glioblastoma to superselective intraarterial cerebral infusion of bevacizumab: A proton MR spectroscopic imaging study. Am J Neuroradiol. 2012. 33: 2095-102
14. Joshi BH, Leland P, Asher A, Prayson RA, Varricchio F, Puri RK. In situ expression of interleukin-4 (IL-4) receptors in human brain tumors and cytotoxicity of a recombinant IL-4 cytotoxin in primary glioblastoma cell cultures. Cancer Res. 2001. 61: 8058-61
15. Kontopodis E, Kanli G, Manikis GC, Van Cauter S, Marias K. Assessing treatment response through generalized pharmacokinetic modeling of DCE-MRI data. Cancer Inform. 2015. 14: 41-51
16. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018. 392: 432-46
17. Lee SJ, Kim JH, Kim YM, Lee GK, Lee EJ, Park IS. Perfusion MR imaging in gliomas: comparison with histologic tumor grade. Korean J Radiol. 2001. 2: 1-7
18. Liu XJ, Duan CF, Fu WW, Niu L, Li Y, Sui QL. Correlation between magnetic resonance perfusion weighted imaging of radiation brain injury and pathology. Genet Mol Res. 2015. 14: 16317-24
19. Metz MC, Molina-Romero M, Lipkova J, Gempt J, LiescheStarnecker F, Eichinger P. Predicting glioblastoma recurrence from preoperative MR scans using fractional-anisotropy maps with free-water suppression. Cancers (Basel). 2020. 12: 728
20. Mohan S, Chawla S, Wang S, Verma G, Skolnik A, Brem S. Assessment of early response to tumor-treating fields in newly diagnosed glioblastoma using physiologic and metabolic MRI: Initial experience. CNS Oncol. 2016. 5: 137-44
21. Mohan S, Wang S, Coban G, Kural F, Chawla S, O’Rourke DM. Detection of occult neoplastic infiltration in the corpus callosum and prediction of overall survival in patients with glioblastoma using diffusion tensor imaging. Eur J Radiol. 2019. 112: 106-11
22. Muruganandham M, Clerkin PP, Smith BJ, Anderson CM, Morris A, Capizzano AA. 3-Dimensional magnetic resonance spectroscopic imaging at 3 Tesla for early response assessment of glioblastoma patients during external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2014. 90: 181-9
23. Puri S, Joshi BH, Sarkar C, Mahapatra AK, Hussain E, Sinha S. Expression and structure of interleukin 4 receptors in primary meningeal tumors. Cancer. 2005. 103: 2132-42
24. Rainov NG, Heidecke V. Long term survival in a patient with recurrent malignant glioma treated with intratumoral infusion of an IL4-targeted toxin (NBI-3001). J Neurooncol. 2004. 66: 197-201
25. Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000. 6: 2157-65
26. Saleh A, Stahopoulou MG, Dade S, Ndiaye NC, AzimiNezhad M, Murray H. Angiogenesis related genes NOS3, CD14, MMP3, and IL4R are associated to VEGF gene expression and circulating levels in healthy adults. BMC Med Genet. 2015. 16: 90
27. Sampson J, Achrol AS, Aghi MK, Bankiewicz K, Bexon M, Brem S.editors. MDNA55 for recurrent glioblastoma (rGBM): targeting the interleukin-4 receptor (IL4R). Am Soc Clin Oncol. 2020. p.
28. Saraswathy S, Crawford FW, Lamborn KR, Pirzkall A, Chang S, Cha S. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J Neurooncol. 2009. 91: 69-81
29. Schmainda KM, Prah M, Connelly J, Rand SD, Hoffman RG, Mueller W. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014. 16: 880-8
30. Verma G, Chawla S, Mohan S, Wang S, Nasrallah M, Sheriff S. Three-dimensional echo planar spectroscopic imaging for differentiation of true progression from pseudoprogression in patients with glioblastoma. NMR Biomed. 2019. 32: e4042
31. Wang S, Kim S, Chawla S, Wolf RL, Zhang WD, O’Rourke DM. Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuro Image. 2009. 44: 653-60
32. Wang S, Martinez-Lage M, Sakai Y, Chawla S, Kim SG, Alonso-Basanta M. Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. Am J Neuroradiol. 2016. 37: 28-36
33. Wang S, O’Rourke DM, Chawla S, Verma G, Nasrallah MP, Morrissette JJ. Multiparametric magnetic resonance imaging in the assessment of anti-EGFRvIII chimeric antigen receptor T cell therapy in patients with recurrent glioblastoma. Br J Cancer. 2019. 120: 54-6
34. Weber F, Asher A, Bucholz R, Berger M, Prados M, Chang S. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003. 64: 125-37