- Department of Neurosurgery, Children’s Health Ireland at Temple St, Dublin, Ireland.
Correspondence Address:
Retaj Mohammad, Department of Neurosurgery, Children’s Health Ireland at Temple St, Dublin, Ireland.
DOI:10.25259/SNI_249_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Retaj Mohammad, Darach Crimmins. Multiple Abscesses in the Frontal, Temporal and Brainstem regions in a 4.5-year-Old Girl- An Illustrative Case Report. 16-Jun-2023;14:209
How to cite this URL: Retaj Mohammad, Darach Crimmins. Multiple Abscesses in the Frontal, Temporal and Brainstem regions in a 4.5-year-Old Girl- An Illustrative Case Report. 16-Jun-2023;14:209. Available from: https://surgicalneurologyint.com/surgicalint-articles/12363/
Abstract
Background: Brainstem located abscesses are rare in the pediatric population. Diagnosis of brain abscess can be challenging as patients may present with nonspecific symptoms and the classical triad of headache, fever, and focal neurological deficit is not always present. Treatment can be conservative or a combination of surgical intervention with antimicrobial therapy.
Case Description: We present the first case of a 4.5-year-old girl with acute lymphoblastic leukemia that developed infective endocarditis (IE) and subsequently developed 3 suppurative collections (frontal, temporal, and brainstem). The patient had negative cerebrospinal, blood, and pus culture growth and subsequently underwent burr-hole drainage of the frontal and temporal abscesses with a 6-week course of intravenous antibiotic therapy with an uneventful postoperative course. At 1 year, the patient is left with minor right lower limb hemiplegia and no cognitive sequelae.
Conclusion: The decision to surgically intervene for brainstem abscesses is dependent on surgeon and patient factors including the presence of multiple collections, midline shift, the aim of source identification in sterile cultures, and the patient’s neurological condition. Patients with hematological malignancies should be monitored closely for IE which is a risk factor for hematogenous spread of brainstem located abscesses.
Keywords: Acute lymphoblastic leukemia, Brainstem abscess, Burr hole, Pediatric
INTRODUCTION
Brain abscess (BA) is a focal suppurative intracranial infection characterized by the formation of a well-define capsule involving the brain parenchyma.[
Diagnosis of BA can be challenging due to the wide range of symptoms children which may present with. The classical triad of headache, fever, and a focal neurological deficit (FND) is only observed in up to 28% of cases.[
The spread of infection may be contiguous or hematogenous. Common predisposing factors in childhood include congenital heart disease and immunosuppression.[
The optimal management remains a controversial topic widely debated by physicians. Treatment can be conservative with antibiotic therapy or may involve surgical intervention.[
Prognosis and outcomes of BA have improved with new advancements in neuroimaging modalities, broadening of the vaccination programs in children, novel antibiotics with improved bacterial cover and blood brain barrier (BBB) penetration, and new surgical techniques.[
To the best of our knowledge, there have been only 27 published cases of pediatric brainstem abscesses.[
CASE PRESENTATION
A 4.5-year-old girl presented to the emergency department with a 2-day history of progressive fatigue, fever, and tachycardia. The patient was diagnosed with ALL 4 months prior and was receiving chemotherapy and following up with the oncology team. The patient’s Glasgow coma scale (GCS) on admission was 15/15 and systems examination demonstrated tachycardia but otherwise unremarkable.
Investigations
Initial blood work showed leukopenia with a white cell count (WCC) of 1.4 × 109 L (4–13.5) and an elevated C-reactive protein (CRP) of 25 mg/L (<5). All other parameters were unremarkable.
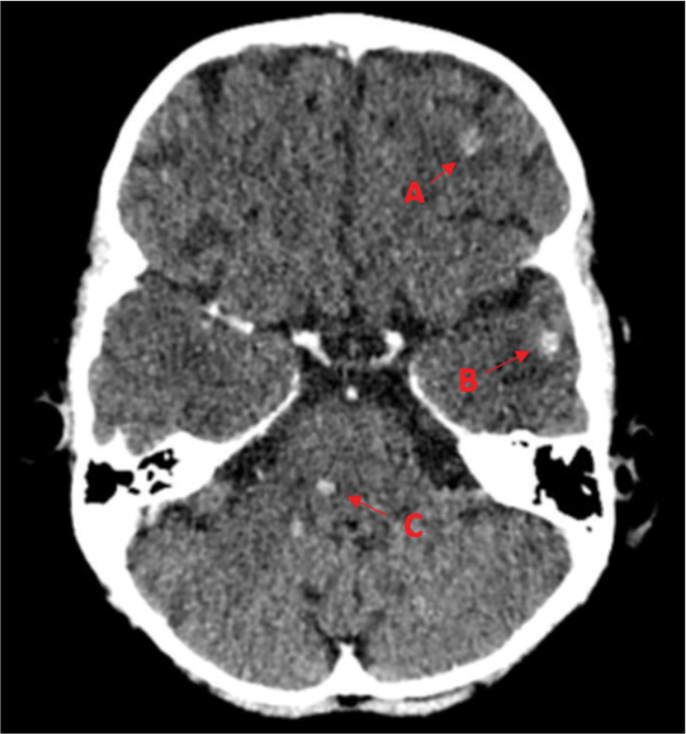
Due to the patient’s cardiac symptoms, an echocardiogram was requested. The echocardiogram revealed vegetations and a diagnosis of infective endocarditis (IE) was made. The patient underwent CT [
Laboratory procedures
A lumbar puncture was performed but both the cerebrospinal fluid (CSF) cultures and blood cultures were negative. After 2 days of admission to the neurosurgical department, the decision for surgical intervention was made due to sterile cultures and difficulty to obtain source control as well as worsening neurological symptoms where the patient started to develop right lower limb weakness. The patient subsequently underwent a burr-hole drainage of the frontal and temporal collections using frameless navigation. This was done using an open right frontal technique with two burr holes and there was no intraoperative imaging during the procedure. Pus culture was aspirated but was also sterile with no growth. The patient was commenced on PiperacillinTazobactam and Vancomycin IV with Amphotericin B and Voriconazole for fungal cover. The patient underwent a total of 6-week duration IV-only antibiotic therapy. The patient also received prednisolone steroid therapy. Midazolam was prescribed as required for seizure prophylaxis. Following the burr-hole aspiration, the patient had an uneventful postoperative course with no surgical complications or re-operation. Postoperative CT scan showed no evidence of hemorrhage and resolution of the midline shift and vasogenic edema. The Glasgow outcome scale (GOS) at discharge was 3/5 due to moderate right lower limb hemiplegia.
At the 1-year follow-up, the patient still suffered from mobility impairment requiring a wheelchair for transportation for long distances. However, GOS at the 1-year follow-up was 4/5 due to minor right lower limb hemiplegia which was an improvement from the GOS at discharge (3/5) as the patient had some improved motor function and required minor assistance with daily activities. The patient was not academically delayed and did not have intellectual disability. For the European Quality 5D Youth quality of life questionnaire, the patient reported “a lot of problems” with mobility and “some problems” with pain and discomfort occasionally requiring painkillers. The European Quality Visual Analog Scale for this patient was 70%. Otherwise, the patient reported no problems with self-care, usual activities, and personality and mood disturbance.
DISCUSSION
Clinical presentation of BRs in the pediatric population is often nonspecific and vague. The classical triad of fever, headache, and FND has been observed in up to 28% of cases and, therefore, is an unreliable indicator.[
Multiple BAs vary in the literature and have been reported in up to 50% of cases in children.[
Laboratory parameters such as an elevated WCC and inflammatory markers including CRP or erythrocyte sedimentation rate may indicate an infectious etiology. However, they are unreliable in the diagnosis of BA as they may be normal in up to 40% of cases.[
Initial microbiological cultures for CSF and blood cultures were negative for any growth. Yogev and Bar-Meir estimate a positive growth rate of 10% of blood cultures in the setting of BA.[
The patient was commenced on Piperacillin-Tazobactam and Vancomycin IV and Amphotericin B and Voriconazole for fungal cover. The patient underwent a total of 6-week duration IV antibiotic therapy. Broad-spectrum antimicrobial therapy should be further narrowed after organism identification and sensitivity reporting. It is challenging to determine the appropriate empiric regimen in patients with ALL when cultures are sterile. Due to the likelihood of fungal infections in this cohort, antifungal agents should always be included. The patient received steroid therapy for cerebral edema and a midline shift observed on MRI scans. There are no clear indications for steroid therapy in BA and it should be used cautiously due to the potential harmful side effects, interference with pathophysiology of abscess formation and impact on antimicrobial BBB penetrance.[
Surgical indication for brainstem abscesses is debatable with various opinions expressed by different surgeons and institutions. Successful nonsurgical treatment of pediatric brainstem abscesses has been reported.[
The aim of source identification was not achieved as pus culture was sterile as well. The patient continued on the same empiric regimen for a total of 6 weeks. However, the GOS outcome was improved at the 1 year follow-up compared to discharge, 4/5 and 3/5, respectively. At the discharge mark, the patient had significant mobility impairment and reduced power in the right lower limb whereas after 1 year, the patient was semi-independent and had minor limb weakness.
There is no difference in outcome in the published literature when comparing different techniques such as burr-hole drainage, endoscopic drainage, or craniotomy. Minimally invasive techniques are preferred as they are safer than an open surgical approach.[
The prognosis of BA has improved over the past five decades with the advancements of neuroimaging and microbiological culture techniques. Before the CT era, the mortality of children with BA was remarkably high at 40–60% and since has dropped to below 10%.[
CONCLUSION
We present the first case to our knowledge, of a 4.5-year-old girl with ALL and multiple collections including a brainstem abscess. The decision to surgically intervene for brainstem abscesses is dependent on surgeon and patient factors including the presence of multiple collections, midline shift, the aim of source identification in sterile cultures, and the patient’s neurological condition. In our case, the frontal and temporal collections were aspirated instead of the smaller brainstem lesion in an effort to obtain source control and improve the neurological deficits. Despite sterile microbiological cultures, this patient recovered well with minor right hemiplegia at 1 year follow-up. There is no superior surgical technique for brainstem located abscesses. Further studies should look at long-term outcomes and empiric regimens in the immunocompromised patient demographic.
Ethical approval
Informed consent was obtained from the patients’ guardian. Ethical approval and data protection agreement were obtained from the Research Ethics Committee of Temple St Children’s University Hospital, Dublin, Ireland (REC-055–21).
Authors’ contributions
Retaj Mohammad was involved in study design, ethical approval and consent seeking, data collection, and analysis and the writing of the manuscript. Darach Crimmins was involved in study design and overall supervision of manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors are grateful to the patient and their family for providing follow-up information and allowing this case to be shared.
References
1. Antkowiak Ł, Putz M, Mandera M. Clinical features, microbiology, and management of pediatric brainstem abscess. Childs Nerv Syst. 2020. 36: 2919-26
2. Arzoglou V, D’Angelo L, Koutzoglou M, Di Rocco C. Abscess of the medulla oblongata in a toddler: Case report and technical considerations based on magnetic resonance imaging tractography. Neurosurgery. 2011. 69: E483-6
3. Atiq M, Ahmed US, Allana SS, Chishti KN. Brain abscess in children. Indian J Pediatr. 2006. 73: 401-4
4. Bodilsen J, Dalager-Pedersen M, van de Beek D, Brouwer MC, Nielsen H. Incidence and mortality of brain abscess in Denmark: A nationwide population-based study. Clin Microbiol Infect. 2020. 26: 95-100
5. Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: Systematic review and meta-analysis. Neurology. 2014. 82: 806-13
6. Brouwer MC, Tunkel AR, McKhann GM, van de Beek D. Brain abscess. N Engl J Med. 2014. 371: 447-56
7. Brouwer MC, van de Beek D. Epidemiology, diagnosis, and treatment of brain abscesses. Curr Opin Infect Dis. 2017. 30: 129-34
8. Danziger J, Allen KL, Bloch S. Brain-stem abscess in childhood. Case report. J Neurosurg. 1974. 40: 391-3
9. Dou ZZ, Guo LY, Liu LL, Li MH, Hu HL, Hu B. Clinical characteristics and outcome analysis of 94 children with brain abscess in beijing: A single-center retrospective study. Pediatr Infect Dis J. 2021. 40: 109-15
10. Fuentes S, Bouillot P, Regis J, Lena G, Choux M. Management of brain stem abscess. Br J Neurosurg. 2001. 15: 57-62
11. Ghannane H, Laghmari M, Aniba K, Lmejjati M, Benali SA. Diagnostic and management of pediatric brain stem abscess, a case-based update. Childs Nerv Syst. 2011. 27: 1053-62
12. Hadi Almuhammed H. Pediatric brainstem abscess: A case-based review. Med J Basrah Univ. 2016. 34: 50-5
13. Imai H, Ono N, Zama A, Tamura M. Diagnosis and treatment of brainstem abscess using magnetic resonance imaging and microsurgical aspiration-case report. Neurol Med Chir (Tokyo). 1995. 35: 160-4
14. Jamjoom ZA. Solitary brainstem abscess successfully treated by microsurgical aspiration. Br J Neurosurg. 1992. 6: 249-53
15. Kashiwagi S, Abiko S, Aoki H. Brainstem abscess. Surg Neurol. 1987. 28: 63-6
16. Kim JH, Jung TY, Jung SH, Lee KH, Kim SK. Pediatric brainstem abscess with hemorrhage mimicking diffuse intrinsic pontine glioma: A case report. Childs Nerv Syst. 2015. 31: 2359-62
17. Kozik M, Ozarzewska E. A solitary abscess of the medulla oblongata. Eur Neurol. 1976. 14: 302-9
18. Mameli C, Genoni T, Madia C, Doneda C, Penagini F, Zuccotti G. Brain abscess in pediatric age: A review. Childs Nerv Syst. 2019. 35: 1117-28
19. Messina AV, Guido LJ, Liebeskind AL. Preoperative diagnosis of brain-stem abscess by computerized tomography with survival. Case report. J Neurosurg. 1977. 47: 106-8
20. Mut M, Hazer B, Narin F, Akalan N, Ozgen T. Aspiration or capsule excision? Analysis of treatment results for brain abscesses at single institute. Turk Neurosurg. 2009. 19: 36-41
21. Muzumdar D, Jhawar S, Goel A. Brain abscess: An overview. Int J Surg. 2011. 9: 136-44
22. Nauta HJ, Contreras FL, Weiner RL, Crofford MJ. Brain stem abscess managed with computed tomography-guided stereotactic aspiration. Neurosurgery. 1987. 20: 476-80
23. Pandian JD, Moosa NV, Cherian PJ, Radhakrishnan K. Brainstem abscess complicating tetralogy of Fallot successfully treated with antibiotics alone. Neurol India. 2000. 48: 272-5
24. Prasanna Kumar M, Krishnamurthy S, Venkateswaran VS, Mahadevan S, Lalitha M, Sistla S. Brainstem micro-abscesses caused by Burkholderia pseudomallei in a 10-month-old infant: A case report. Paediatr Int Child Health. 2017. 37: 230-2
25. Raffaldi I, Garazzino S, Gattinara GC, Lipreri R, Lancella L, Esposito S. Brain abscesses in children: An Italian multicentre study. Epidemiol Infect. 2017. 145: 2848-55
26. Rajshekhar V, Chandy MJ. Successful stereotactic management of a large cardiogenic brain stem abscess. Neurosurgery. 1994. 34: 368-71
27. Ramdasi RV, Patil MK, Muzumdar DP. Solitary pyogenic brainstem abscess. Neurol India. 2016. 64: 572-4
28. Robert CM, Stern WE, Brown WJ, Greenfield MA, Bentson JR. Brain stem abscess treated surgically, Wtih special note upon the employment of thorium dioxide. Surg Neurol. 1975. 3: 153-60
29. Russell JA, Shaw MD. Chronic abscess of the brain stem. J Neurol Neurosurg Psychiatry. 1977. 40: 625-9
30. Sadeghpour Tabaei A, Koochakzadeh L, Khorgami M, Tabaei SS. Acute lymphoblastic leukemia with infective endocarditis presented with unusual intracardiac mass. Case Rep Cardiol. 2017. 2017: 1528416
31. Sarmast AH, Showkat HI, Kirmani AR, Bhat AR, Patloo AM, Ahmad SR. Aspiration versus excision: A single center experience of forty-seven patients with brain abscess over 10 years. Neurol Med Chir (Tokyo). 2012. 52: 724-30
32. Shachor-Meyouhas Y, Bar-Joseph G, Guilburd JN, Lorber A, Hadash A, Kassis I. Brain abscess in children-epidemiology, predisposing factors and management in the modern medicine era. Acta Paediatr. 2010. 99: 1163-7
33. Suzer T, Coskun E, Cirak B, Yagci B, Tahta K. Brain stem abscesses in childhood. Childs Nerv Syst. 2005. 21: 27-31
34. van der Velden FJ, Battersby A, Pareja-Cebrian L, Ross N, Ball SL, Emonts M. Paediatric focal intracranial suppurative infection: A UK single-centre retrospective cohort study. BMC Pediatr. 2019. 19: 130
35. Vaquero J, Cabezudo JM, Leunda G. Nonsurgical resolution of a brain-stem abscess. Case report. J Neurosurg. 1980. 53: 726-7
36. Wabi G. Brain stem abscess. Indian Pediatr. 1993. 30: 547-8
37. Wang HS, Kuo MF, Huang SC. Medical cure of a brainstem abscess and serial brainstem auditory evoked potentials. Dev Med Child Neurol. 1992. 34: 911-5
38. Wiswell W, Jerzewski K, McGuire M. A rare case of a medulla oblongata brain abscess in a 23-month-old child. Pediatr Emerg Care Med Open Access. 2019. 4: 1
39. Xu B, Zhang Y, Yu J. Brainstem tuberculous abscesses successfully treated by microsurgical excision: A case report and review of the literature. Oncol Lett. 2017. 13: 2708-12
40. Yogev R, Bar-Meir M. Management of brain abscesses in children. Pediatr Infect Dis J. 2004. 23: 157-9
41. Yoshikawa T, Goodman S. Brain-abscess. West J Med. 1974. 121: 207-19