- Department of Neurosurgery, Saitama Medical Center, Saitama Medical University, Saitama, Japan
- Department of Radiology, Graduate School of Medicine and Faculty of Medicine, University of Tokyo, Tokyo, Japan
Correspondence Address:
Soichi Oya
Department of Neurosurgery, Saitama Medical Center, Saitama Medical University, Saitama, Japan
DOI:10.4103/2152-7806.166799
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tsuchiya T, Oya S, Mori H, Matsui T. Multiple hemorrhagic intraparenchymal tumors presenting with fatal intracranial hypertension: A rare manifestation of systemic epithelioid hemangioendothelioma. Surg Neurol Int 06-Oct-2015;6:156
How to cite this URL: Tsuchiya T, Oya S, Mori H, Matsui T. Multiple hemorrhagic intraparenchymal tumors presenting with fatal intracranial hypertension: A rare manifestation of systemic epithelioid hemangioendothelioma. Surg Neurol Int 06-Oct-2015;6:156. Available from: http://surgicalneurologyint.com/surgicalint_articles/multiple-hemorrhagic-intraparenchymal-tumors-presenting-with/
Abstract
Background:Epithelioid hemangioendotheliomas (EHE) is an extremely rare tumor that can arise not only intracranially but also systemically. Its radiological characteristics and the mechanism underlying the multiple organ involvement in EHE are poorly understood.
Case Description:A 24-year-old woman with a 7-month history of coughing and blood-stained sputum complained of visual disturbance in the right eye that had persisted for 1-month. Magnetic resonance (MR) imaging revealed multiple intraparenchymal masses with low-intensity on MR susceptibility-weighted images with minimal enhancement with gadolinium. Systemic computed tomography revealed multiple nodules in both lungs and the liver. Because her neurological status rapidly deteriorated, brain biopsy of the right frontal mass was performed. The pathological diagnosis was EHE. Over the following 3 months, the patient gradually developed disturbance of consciousness. She died at 4 months after admission because of significant intracranial hypertension.
Conclusion:Although intracranial EHEs are extremely rare, they should be included in the differential diagnoses of multiple small-sized masses with low-intensity on MR susceptibility-weighted images. We also emphasize that the systemic involvement of this tumor was more compatible with multicentric development than metastasis.
Keywords: Epithelioid hemangioendothelioma, hemorrhagic brain tumor, liver tumor, multiple intracranial mass, pulmonary tumor
INTRODUCTION
Epithelioid hemangioendothelioma (EHE) is a rare tumor of vascular endothelial origin with an epithelioid appearance.[
We describe a case of a 24-year-old female who presented with myriad intracranial small lesions resembling hematomas on computed tomography (CT) scans and magnetic resonance images (MRIs). She was also found to have similar small lesions in the lungs and liver. Although the histology of the biopsied specimen showed benign features with a low proliferation index, her clinical course was very rapid due to intracranial hypertension caused by multiple intracranial masses.
CASE REPORT
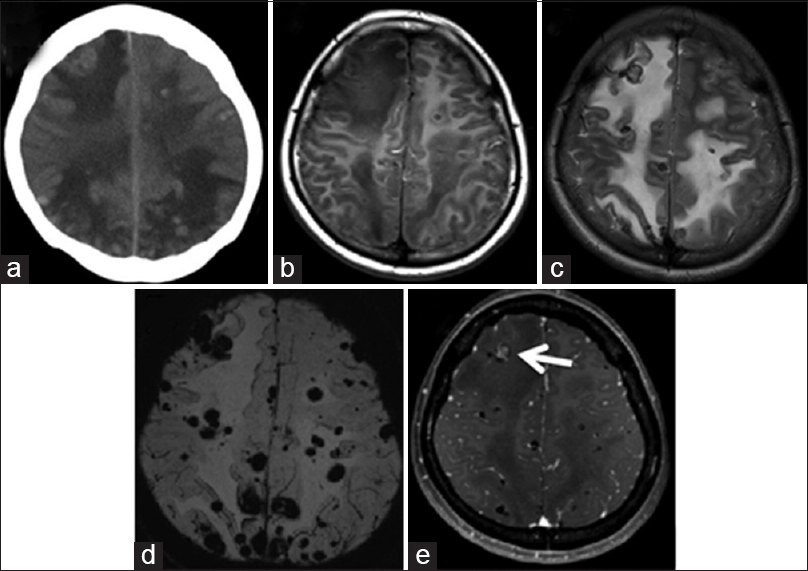
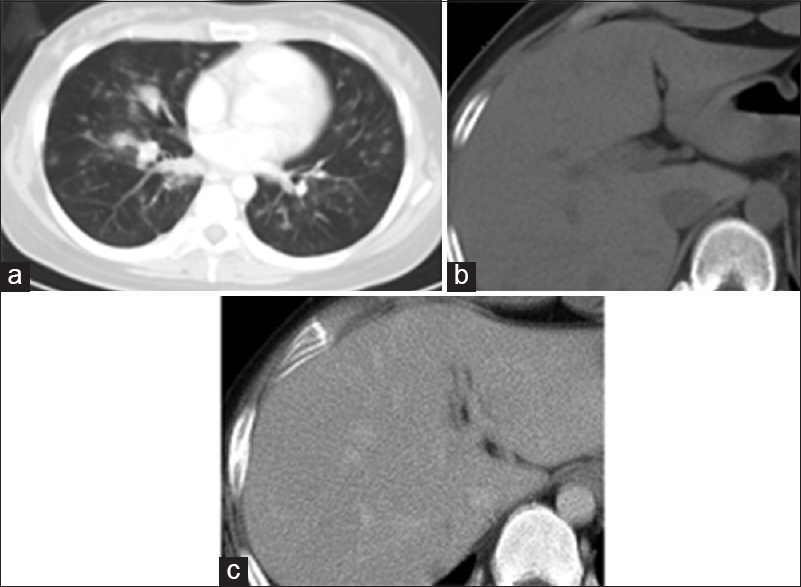
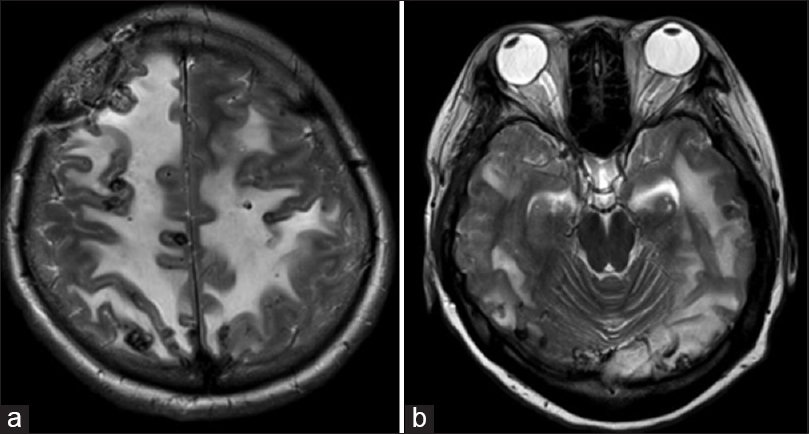
A 24-year-old woman with no remarkable previous medical history had a 7-month history of coughing and blood-stained sputum. She also presented with a visual disturbance in the right eye occurring over 1-month. She visited a local ophthalmologist and was referred to an ophthalmologist at our hospital. Detailed examinations revealed no ophthalmological abnormality. Brain MRI showed multiple intracranial lesions and she was referred to us. On examination, she was awake and alert with no disorientation except for visual deficits rated as counting fingers in the right eye, whereas the left visual acuity was 30/50. There were no other focal neurological deficits. A brain CT scan revealed multiple 5–15 mm high-density nodules with perifocal edema in the bilateral cerebrum and cerebellum [
Figure 1
(a) Brain computed tomography scan showed multiple small intra-axial nodules with slightly high density. (b) Magnetic resonance imaging demonstrated that the signal of the nodules was hypointense to isointense on the T1-weighted image. (c) The magnetic resonance T2-weighted image revealed significant edema around multiple nodular lesions showing hypointensity. (d) The magnetic resonance susceptibility-weighted image showed numerous low-intensity spots, indicating old hemorrhages. (e) Magnetic resonance T1-weighted image with gadolinium enhancement demonstrated little enhancement in most lesions but a weak enhancement in some nodules (arrow)
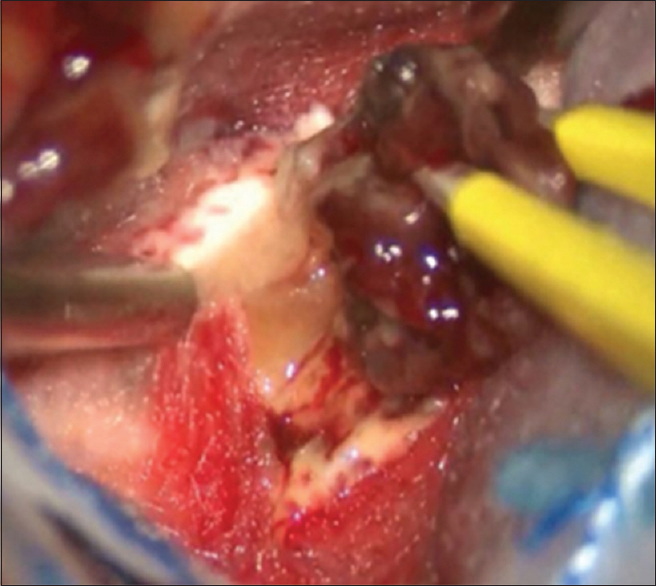
During the course of these examinations, the patient's condition significantly progressed. Her bilateral visual acuity rapidly declined for 3 days after admission. She also had frequent general clonic seizures. The steroid, osmotic diuretics, carbamazepine, and levetiracetam were administered to control her seizures. Although her seizures were controlled over the following 3 days, her bilateral visual acuity had further declined to light perception. Because of the necessity of determining pathological diagnosis, a biopsy of the right frontal brain lesion was performed 14 days after admission. Increased intracranial pressure was observed intraoperatively. The lesion with slight enhancement on MRIs in the right frontal lobe was removed using a navigation guide. The tumor was moderately hemorrhagic, presenting a reddish-brown color [
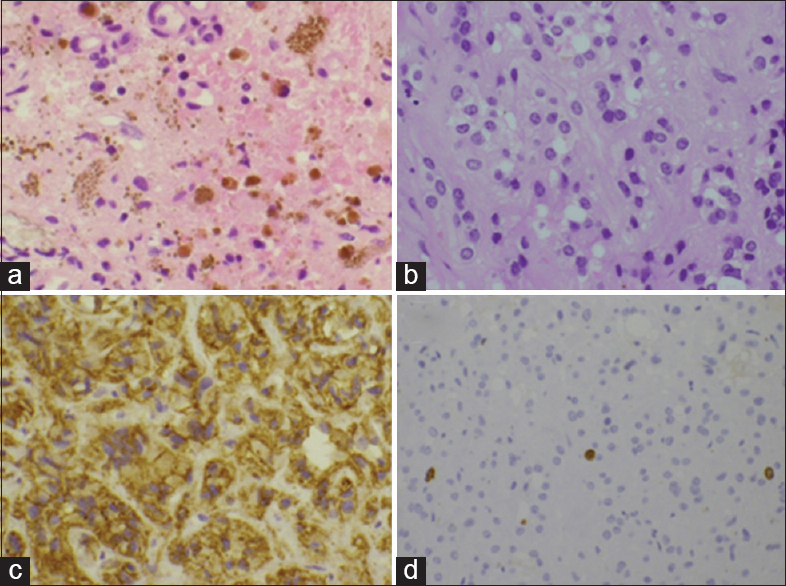
Histopathological examination showed a diffuse cellular proliferation upon hematoxylin and eosin stain. Fine vascular channels, hemorrhage, and hemosiderosis were observed in the tissue [
Figure 4
(a) Hematoxylin and eosin staining showing diffuse cellular proliferation, fine vascular channels, hemorrhage, and hemosiderosis. (b) Cells with a round and slightly coarse nucleus and a large volume of clear cytoplasm including large and small balloon-like lesions and erythrocytes were noted. (c) Immunostaining for CD31 revealed a significant staining of the cell membrane and cytoplasm. (d) The MIB-1 labeling index was <3% in the tumor cells
The patient's conditions were stable for 3 months after biopsy. After a discussion about the treatment strategy with the patient and her family, we opted for conservative therapy comprising only rehabilitation on the basis of the low proliferation rate indicated by the histopathological findings. However, after 3 months, the patient gradually developed moderate disturbance of consciousness, headache, and vomiting. MRIs demonstrated aggravation of peritumoral edema without apparent enlargement of each nodule [
The autopsy revealed the cause of death as brainstem necrosis, possibly induced by brain herniation. In addition to the multiple intracranial, pulmonary, and hepatic lesions confirmed on diagnostic imaging, multiple white nodules were observed, including two in the spleen, two in the left kidney, one in the right kidney, and one in the third lumbar vertebra. All were confirmed as EHE.
DISCUSSION
The term EHE was coined by Weiss and Enzinger in 1982 to designate a vascular tumor with an epithelioid appearance.[
On radiological examination, intracranial EHE can be extra- or intra-axial, with a variable size.[
The mechanism underlying the multiple organ involvement in EHE is poorly understood. Two hypotheses have been proposed; metastasis[
The clinical course of EHE is also variable. Some reports describe cases with no progression after a long follow-up period,[
Although the treatment strategy for EHE has not been well-established because of its rarity, most previous reports advocate surgical resection for intracranial lesions that are solitary and completely resectable.[
CONCLUSION
EHE is rare and can present as solitary or multiple intracranial masses. Although the diagnosis is not simple, radiological features indicating a mixture of old and new hemorrhages inside tumors are helpful. It should be included in the differential diagnoses, particularly when multiple intracranial, pulmonary, and hepatic lesions are observed. The systemic involvement of this tumor was more compatible with multicentric development than metastasis. The clinical manifestation of EHE can be rapid and aggressive regardless of a low proliferative potential.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aniba K, Laghmari M, Lmejjati M, Ghannane H, Ait Benali S. A tragical paediatric case history of intraorbital and intracranial epithelioid hemangioendothelioma. Case Rep Neurol Med 2012. 2012. p.
2. Baehring JM, Dickey PS, Bannykh SI. Epithelioid hemangioendothelioma of the suprasellar area: A case report and review of the literature. Arch Pathol Lab Med. 2004. 128: 1289-93
3. Chan YL, Ng HK, Poon WS, Cheung HS. Epithelioid haemangioendothelioma of the brain: A case report. Neuroradiology. 2001. 43: 848-50
4. Chen TC, Gonzalez-Gomez I, Gilles FH, McComb JG. Pediatric intracranial hemangioendotheliomas: Case report. Neurosurgery. 1997. 40: 410-4
5. Chow LT, Chow WH, Fong DT. Epithelioid hemangioendothelioma of the brain. Am J Surg Pathol. 1992. 16: 619-25
6. d’Annibale M, Piovanello P, Carlini P, Del Nonno F, Sciarretta F, Rossi M. Epithelioid hemangioendothelioma of the liver: Case report and review of the literature. Transplant Proc. 2002. 34: 1248-51
7. Díaz R, Segura A, Calderero V, Cervera I, Aparicio J, Jordá MV. Central nervous system metastases of a pulmonary epitheloid haemangioendothelioma. Eur Respir J. 2004. 23: 483-6
8. Endo T, Su CC, Numagami Y, Shirane R. Malignant intracranial epithelioid hemangioendothelioma presumably originating from the lung: Case report. J Neurooncol. 2004. 67: 337-43
9. Fernandes AL, Ratilal B, Mafra M, Magalhaes C. Aggressive intracranial and extra-cranial epithelioid hemangioendothelioma: A case report and review of the literature. Neuropathology. 2006. 26: 201-5
10. Fryer JA, Biggs MT, Katz IA, Brazier DH, Shakespeare TP. Intracranial epithelioid hemangioendothelioma arising at site of previously excised atypical meningioma. Pathology. 1998. 30: 95-9
11. Gelin M, Van de Stadt J, Rickaert F, De Prez C, Levarlet M, Adler M. Epithelioid hemangioendothelioma of the liver following contact with vinyl chloride. Recurrence after orthotopic liver transplantation. J Hepatol. 1989. 8: 99-106
12. Golash A, Strang FA, Reid H. Intracranial haemangioendothelioma mimicking a meningioma. Br J Neurosurg. 1999. 13: 594-7
13. Hamlat A, Casallo-Quilliano C, Saikali S, Lesimple T, Brassier G. Epithelioid hemangioendothelioma of the infundibular-hypothalamic region: Case report and literature review. J Neurooncol. 2004. 67: 361-6
14. Hodaie M, Becker L, Teshima I, Rutka JT. Total resection of an intracerebral hemangioendothelioma in an infant. Case report and review of the literature. Pediatr Neurosurg. 2001. 34: 104-12
15. Hurley TR, Whisler WW, Clasen RA, Smith MC, Bleck TP, Doolas A. Recurrent intracranial epithelioid hemangioendothelioma associated with multicentric disease of liver and heart: Case report. Neurosurgery. 1994. 35: 148-51
16. Kitaichi M, Nagai S, Nishimura K, Itoh H, Asamoto H, Izumi T. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J. 1998. 12: 89-96
17. Kubota T, Sato K, Takeuchi H, Handa Y. Successful removal after radiotherapy and vascular embolization in a huge tentorial epithelioid hemangioendothelioma: A case report. J Neurooncol. 2004. 68: 177-83
18. Llena JF, Hirano A, Inoue A. Vasoformative tumor of the brain – Immunohistology and ultrastructure. Clin Neuropathol. 1984. 3: 155-9
19. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
20. Mistry AM, Gorden DL, Busler JF, Coogan AC, Kelly BS. Diagnostic and therapeutic challenges in hepatic epithelioid hemangioendothelioma. J Gastrointest Cancer. 2012. 43: 521-5
21. Mohan S M, Symss NP, Pande A, Chakravarthy VM, Ramamurthi R. Intracranial epithelioid hemangioendothelioma. Childs Nerv Syst. 2008. 24: 863-8
22. Nora FE, Scheithauer BW. Primary epithelioid hemangioendothelioma of the brain. Am J Surg Pathol. 1996. 20: 707-14
23. Parajón A, Vaquero J. Meningel intracranial epithelioid hemangioendothelioma: Case report and literature review. J Neurooncol. 2008. 88: 169-73
24. Pearl GS, Takei Y, Tindall GT, O’Brien MS, Payne NS, Hoffman JC. Benign hemangioendothelioma involving the central nervous system: “Strawberry nevus” of the neuraxis. Neurosurgery. 1980. 7: 249-56
25. Phookan G, Davis AT, Holmes B. Hemangioendothelioma of the cavernous sinus: Case report. Neurosurgery. 1998. 42: 1153-5
26. Puca A, Meglio M, Rollo M, Zannoni GF. Intracranial epithelioid hemangioendothelioma: Case report. Neurosurgery. 1996. 38: 399-401
27. Radin DR, Craig JR, Colletti PM, Ralls PW, Halls JM. Hepatic epithelioid hemangioendothelioma. Radiology. 1988. 169: 145-8
28. Rocha Oliveira PC, Alcantara FP, Souza-Vianna PE, Brito AP. Cerebral epithelioid hemangioendothelioma with thoracic simultaneous involvement: Advanced MRI features. Arq Neuropsiquiatr. 2012. 70: 637-8
29. Rushing EJ, White JA, D’ Alise MD, Chason DP, White CL, Bigio EH. Primary epithelioid hemangioendothelioma of the clivus. Clin Neuropathol. 1998. 17: 110-4
30. Samaratunga H, Searle J, Cominos D, Le Fevre I. Cerebral metastasis of an atrial myxoma mimicking an epithelioid hemangioendothelioma. Am J Surg Pathol. 1994. 18: 107-11
31. Sumrall A, Fredericks R, Berthold A, Shumaker G. Lenalidomide stops progression of multifocal epithelioid hemangioendothelioma including intracranial disease. J Neurooncol. 2010. 97: 275-7
32. Tammam AG, Lewis PD, Crockard HA. Cerebello-pontine angle epithelioid haemangioendothelioma in a 4-year-old boy. Childs Nerv Syst. 1997. 13: 648-50
33. Tancredi A, Puca A, Carbone A. Multifocal cerebral hemangio-endothelioma. Case report and review of the literature. Acta Neurochir. 2001. 142: 1157-61
34. Taratuto AL, Zurbriggen G, Sevlever G, Saccoliti M. Epithelioid hemangioendothelioma of the central nervous system. Immunohistochemical and ultrastructural observations of a pediatric case. Pediatr Neurosci. 1988. 14: 11-4
35. Venizelos ID, Paradinas FJ. Primary paediatric intracranial epithelioid haemangioendothelioma. Histopathology. 2002. 41: 172-4
36. Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: A vascular tumor often mistaken for a carcinoma. Cancer. 1982. 50: 970-81
37. Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol. 1986. 3: 259-87
38. Wong DS, Chiu TW, Wong GK, Zhu XL, Kwok MW, Ho CM. Epithelioid haemangioendothelioma of the anterior skull base: What is the optimal treatment?. Hong Kong Med J. 2009. 15: 308-10
39. Ye B, Li W, Feng J, Shi JX, Chen Y, Han BH. Treatment of pulmonary epithelioid hemangioendothelioma with combination chemotherapy: Report of three cases and review of the literature. Oncol Lett. 2013. 5: 1491-1496
40. Zhang J, Wang Y, Geng D. Intracranial epithelioid hemangioendothelioma: An unusual CTA finding in one case. Br J Neurosurg. 2010. 24: 294-5
41. Zheng J, Liu L, Wang J, Wang S, Cao Y, Zhao J. Primary intracranial epithelioid hemangioendothelioma: A low-proliferation tumor exhibiting clinically malignant behavior. J Neurooncol. 2012. 110: 119-27