- Department of Neurosurgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
Correspondence Address:
Rahul Raj
Department of Neurosurgery, University of Helsinki and Helsinki University Hospital, Helsinki, Finland
DOI:10.4103/sni.sni_22_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Rahul Raj, Miikka Korja, Päivi Koroknay-Pál, Mika Niemelä. Multiple meningiomas in two male-to-female transsexual patients with hormone replacement therapy: A report of two cases and a brief literature review. 25-May-2018;9:109
How to cite this URL: Rahul Raj, Miikka Korja, Päivi Koroknay-Pál, Mika Niemelä. Multiple meningiomas in two male-to-female transsexual patients with hormone replacement therapy: A report of two cases and a brief literature review. 25-May-2018;9:109. Available from: http://surgicalneurologyint.com/surgicalint-articles/multiple-meningiomas-in-two-male%e2%80%91to%e2%80%91female-transsexual-patients-with-hormone-replacement-therapy-a-report-of-two-cases-and-a-brief-literature-review/
Abstract
Background:Exogenous sex hormones may play a role in meningioma development and growth. Thus, transsexual patients being on long-standing hormone replacement therapy (HRT) may be at particular risk for meningioma development and growth. Here we present two cases of two male-to-female transsexual patients taking HRT for an extended period of time with both patients requiring surgical treatment at our institution due to multiple growing meningiomas.
Case Description:The first patient was a 50-year-old genetic male (male-to-female transsexual) who presented 7 years after an extensive sex-change operation due to progressive bitemporal visual defects. The patient had been on HRT for approximately a decade. Radiological examinations showed a total of four meningiomas, one being a large suprasellar meningioma causing the symptoms. Three of the four meningiomas were operated on, but the patient's vision could not be saved. Immunohistochemical (IH) analysis of the meningiomas showed both estrogen and progesterone receptor expression. The patient continued HRT and during follow-up regrowth of the meningiomas was noted. The second patient was a 48-year-old genetic male (male-to-female transsexual) who had been on HRT for two decades and also presented due to left-sided visual loss. Radiological examination showed four meningiomas, one being a left-sided sinus cavernous meningioma causing compression of the visual apparatus. This patient had a previous normal head computerized tomography scan dating back 10 years before his current presentation. Three of the four meningiomas were operated on with a slight improvement in visual acuity. IH analysis showed positive progesterone receptor expression but negative estrogen receptor expression.
Conclusion:Radiological screening may be justifiable for transsexual patients with a history of long-standing HRT and special focus should be paid to transsexual patients displaying new neurological symptoms or those already diagnosed with a meningioma.
Keywords: Estrogen, hormone replacement therapy, meningioma, progesterone, transsexual
INTRODUCTION
The role of sex hormones as a risk factor for meningioma development and growth has been a topic of debate.[
Here we report two further cases of transsexual patients with long-standing HRT developing several meningiomas and a brief overview of the literature on this topic.
CASE REPORTS
Case 1
A 43-year-old genetic male underwent a male-to-female sex-change operation. The patient had been taking HRT for several years before the sex-change operation. Following surgery, the patient continued HRT, consisting of estradiol cream (0.6 mg/g) twice a day and cyproterone acetate (Andocur®) 50 mg once a day.
This patient presented 7 years later with progressive bilateral visual compromise leading to complete left-sided vision loss. Clinical examination showed normal visual acuity on the right side (20/20). The left eye could only perceive light sensation, and clinical fundus examination revealed left-sided optic disk atrophy, with a dilated pupil and a limited direct pupillary light constriction reflex.
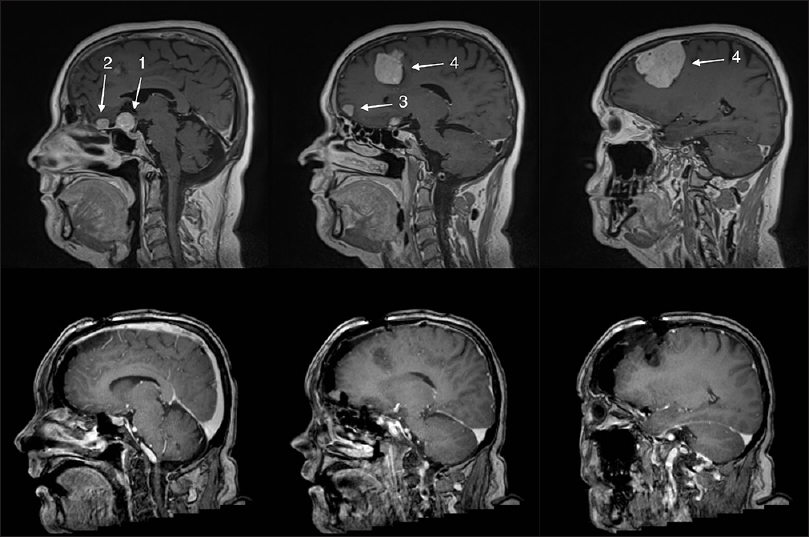
The patient was sent to neuroimaging and magnetic resonance imaging (MRI) showed a total of four meningiomas: (1) a suprasellar meningioma compressing the left optic nerve and chiasm causing the patient's symptoms, (2) a small frontobasal midline meningioma at the level of cribriform plate, (3) a small frontal right-sided convexity meningioma, and another (4) large frontal right-sided convexity meningioma [
Figure 1
Upper row shows the pre-operative magnetic resonance imaging (MRI) showing four meningiomas: (1) a suprasellar meningioma, (2) a small frontal midline meningioma in front of the suprasellar meningioma, (3) a right-sided midline frontal convexity meningioma, and a (4) large right-sided frontal convexity meningioma. Lower row shows the 1-week postoperative MRI where meningiomas (1), (2), and (4) have been totally removed. Numbers 1–4 refer to meningiomas as mentioned in the main text
Meningiomas 1, 2, and 4 (suprasellar, frontobasal midline, frontal right-sided convexity) could all be surgically accessed in one single session with adequate results (Simpson grade III for suprasellar and frontobasal meningiomas and Simpson grade I for convexity meningioma). The histological diagnosis in all these meningiomas revealed World Health Organization (WHO) grade I tumors with low proliferative index (MIB-1 <5%). Further immunohistochemical (IH) profiling revealed a high level of progesterone receptor expression and lower level expression of estrogen receptors.
Immediately after surgery, the limited function that had been present in the left eye remained unchanged, but unfortunately the visual acuity of the right eye acutely deteriorated (to light perception only). Postoperative head computed tomography (CT) showed no intracranial hemorrhage. The patient was taken back to the operating room for exploration. Intraoperative finding showed acute swelling of the right optic nerve, and a right-sided optic canal decompression and optic nerve sheath fenestration were performed. Unfortunately, vision could not be restored in the right eye and the patient became legally blind since both eyes suffered from compromised vision. After close inspection of anesthesiology reports, no hypotensive insult before or during the first or second operation was noted.
Postoperative MRI showed complete removal of (1) suprasellar meningioma, (2) frontobasal midline meningioma, and (4) frontal right-sided convexity meningioma [
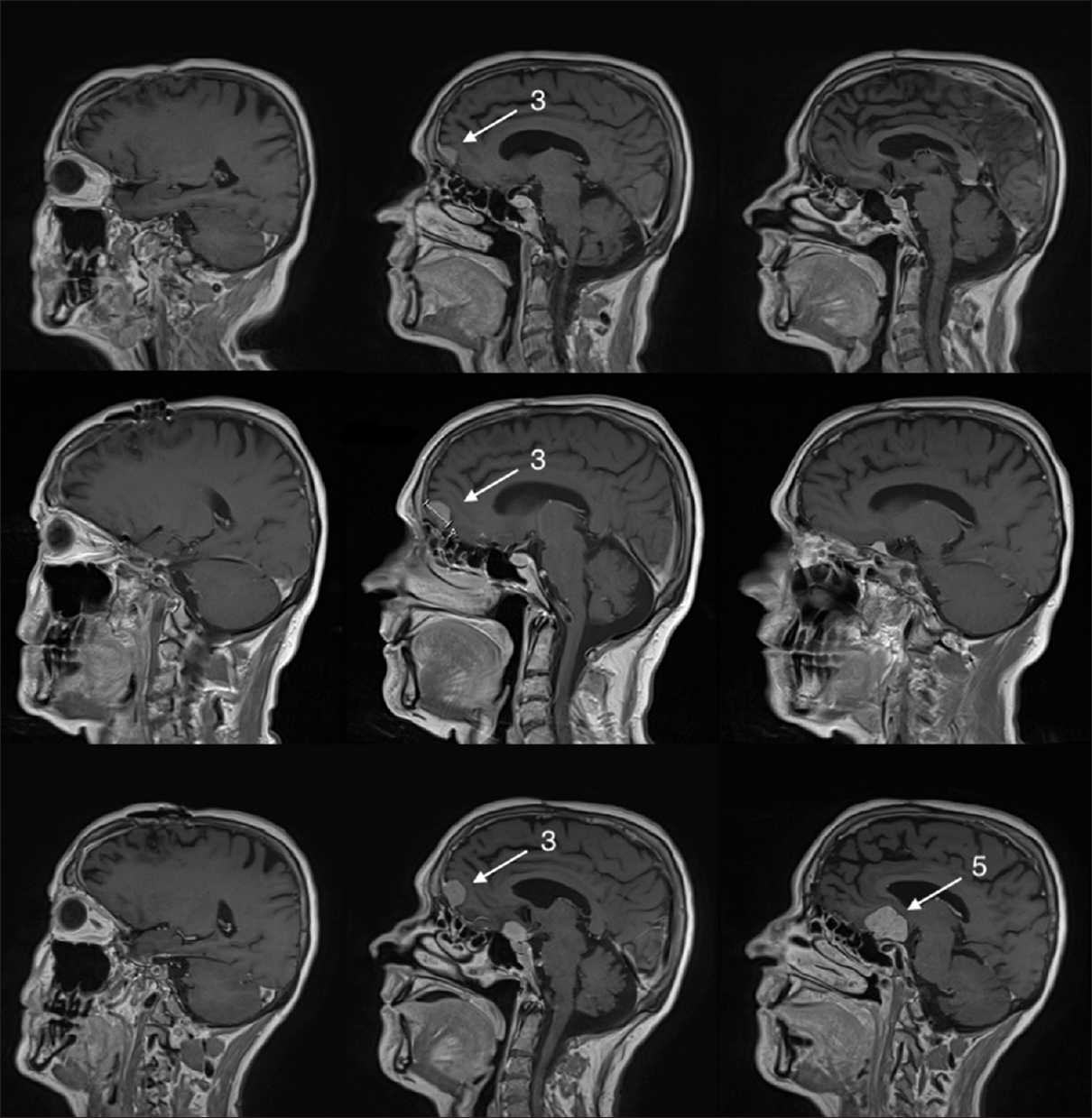
The patient was followed with sequential imaging, and 1 year after surgery a repeat MRI showed no regrowth of resected meningiomas but slight growth of small frontal right-sided midline convexity meningioma (3) that had been left untouched [
Figure 2
Upper row shows the 1-year postoperative magnetic resonance imaging (MRI) with no regrowth of the operated meningiomas (1, 2, 4) and no major growth of the (3) right-sided midline frontal convexity meningioma. Middle row shows the 2-year postoperative MRI with some growth of (3) right-sided midline frontal convexity meningioma and growth of a (5) new meningioma in the suprasellar space. Lower row shows the 4-year postoperative MRI showing some growth of the unoperated (3) right-sided midline frontal convexity meningioma and major growth of the (5) new suprasellar meningioma. Numbers 1–4 refer to meningiomas as mentioned in the main text
At 2 years, postoperative MRI showed regrowth of a (5) new suprasellar meningioma and further growth of unoperated frontal right-sided midline convexity meningioma (3). The patient was managed expectantly and HRT was continued unchanged [
Four years after operation, another MRI showed significant growth of (5) suprasellar meningioma and (3) of the previous unoperated right-sided midline frontal convexity meningioma [
Case 2
This is a 48-year old male-to-female transsexual (genetic male), who had been taking HRT for 21 years before neurosurgical presentation. The HRT consisted of estradiol cream (1 mg/g) three times a day and cyproterone acetate (Andocur) 50 mg once a day.
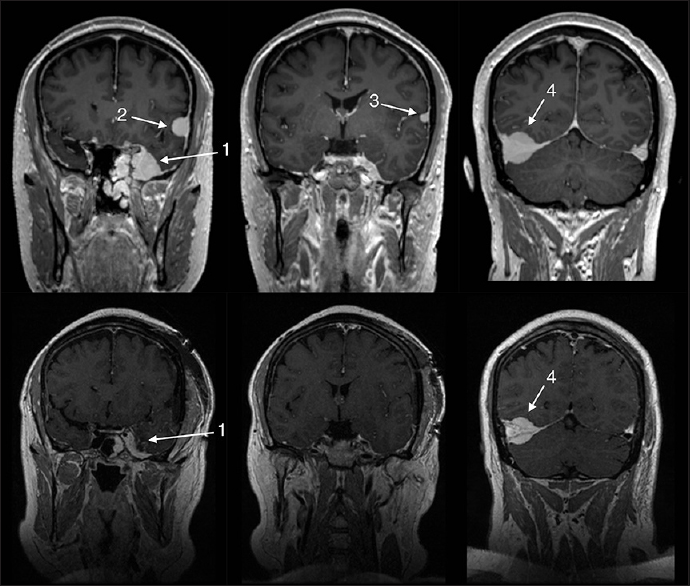
The patient presented with progressive left-sided vision loss. Clinical examination revealed near-normal visual acuity in the right eye (20/20) but extremely compromised visual acuity in the left eye (20/250) with left-sided optic atrophy on fundus examination. A brain MRI was obtained that revealed four meningiomas: (1) in the left-sided sinus cavernous, (2) over the left-sided frontal convexity, (3) over the left-sided temporal convexity, and (4) right-sided tentorial [
Figure 3
Upper row shows the pre-operative magnetic resonance imaging (MRI) revealing a total of four meningiomas: (1) left-sided sinus cavernous, (2) left-sided frontal convexity, (3) left-sided temporal convexity, and (4) right-sided tentorial. Lower row showing immediate postoperative MRI revealing subtotal resection of the (1) sinus cavernous meningioma and total removal of the two left-sided convexity meningiomas (2, 3)
Three of the meningiomas (sinus cavernous, left-sided frontal, and temporal) were operated on and the left optic nerve was decompressed in the same surgical sitting at about 5 months after symptom had started. Surgery was successful and achieved Simpson grade I for convexity meningiomas and Simpson grade IV for sinus cavernous meningioma. Postoperative MRI showed complete removal of convexity meningiomas and a reduction in the size of sinus cavernous meningioma [
It was recommended to the patient to taper down the HRT, but long-term follow-up is still lacking.
DISCUSSION
Here we report our observations of long-term use of HRT and the growth of multiple meningiomas in two man-to-female transsexual patients. In the first patient, growth of several meningiomas was noticed (over a 4-year follow-up following removal) and it seems probable that this is related by the use of exogenous HRT. Since the patient had not undergone any brain imaging before their clinical presentation, we cannot demonstrate the causal role of HRT and risk of meningioma development. However, given the receptor expression profile of the pathological specimen, this explanation appears intuitive. The link between exogenous HRT and meningioma is further supported in the second case, by the fact that the patient had undergone a contrast-enhanced head CT scan 10 years before the discovery of his meningiomas, and that CT was read out as completely normal. Thus, it seems likely that the exogenous HRT had a role in the development and growth of these patients' multiple meningiomas.
Epidemiological studies have revealed that females are more likely to develop meningiomas in the second half of life, and due to the fact that many meningiomas may express progesterone and estrogen receptors, it has been hypothesized that both endogenous and exogenous sex hormones may increase the risk for meningioma development and growth.[
Previous studies have shown an association between HRT and risk of meningioma occurrence in post-menopausal women, especially when taking estradiol supplements only.[
With this report, we are hoping to raise awareness of this problem and to highlight the importance of brain imaging in male-to-female transsexuals displaying new neurologic symptoms that may be related to intracranial processes and especially if individuals have already been diagnosed with meningiomas. Still, further case–control matched epidemiological studies are necessary to determine the real risk of meningioma in transsexual persons.
CONCLUSION
The use of exogenous sex hormones may play a role in the development and growth of meningiomas. The risk may be dose-dependent, which would suggest that risk is especially high in male-to-female transsexual patients taking high doses of exogenous sex hormones over long periods. Radiological screening may be justifiable for transsexual patients with a history of long-standing high-dose exogenous sex-hormone therapy and special focus should be paid to transsexual patients displaying new neurological symptoms or those already diagnosed with a meningioma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Benson VS, Pirie K, Green J, Casabonne D, Beral V. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008. 99: 185-90
2. Benson VS, Kirichek O, Beral V, Green J. Menopausal hormone therapy and central nervous system tumor risk: Large UK prospective study and meta-analysis. Int J Cancer. 2015. 136: 2369-77
3. Benson VS, Pirie K, Green J, Bull D, Casabonne D, Reeves GK. Hormone replacement therapy and incidence of central nervous system tumours in the Million Women Study. Int J Cancer. 2010. 127: 1692-8
4. Cea-Soriano L, Blenk T, Wallander MA, Rodríguez LAG. Hormonal therapies and meningioma: Is there a link?. Cancer Epidemiol. 2012. 36: 198-205
5. Cebula H, Pham TQ, Boyer P, Froelich S. Regression of meningiomas after discontinuation of cyproterone acetate in a transsexual patient. Acta Neurochir (Wien). 2010. 152: 1955-6
6. Christensen HC, Kosteljanetz M, Johansen C. Incidences of gliomas and meningiomas in Denmark, 1943 to 1997. Neurosurgery. 2003. 52: 1327-34
7. Claus EB, Black PM, Bondy ML, Calvocoressi L, Schildkraut JM, Wiemels JL. Exogenous hormone use and meningioma risk. Cancer. 2007. 110: 471-6
8. DeIpolyi AR, Han SJ, Parsa AT. Development of a symptomatic intracranial meningioma in a male-to-female transsexual after initiation of hormone therapy. J Clin Neurosci. 2010. 17: 1324-6
9. Gazzeri R, Galarza M, Gazzeri G. Growth of a meningioma in a transsexual patient after estrogen–progestin therapy. N Engl J Med. 2007. 357: 2411-2
10. Goncalves AMG, Page P, Domigo V, Meder JF, Oppenheim C. Abrupt regression of a meningioma after discontinuation of cyproterone treatment. Am J Neuroradiol. 2010. 31: 1504-5
11. Korhonen K, Auvinen A, Lyytinen H, Ylikorkala O, Pukkala E. A nationwide cohort study on the incidence of meningioma in women using postmenopausal hormone therapy in Finland. Am J Epidemiol. 2012. 175: 309-14
12. Korhonen K, Salminen T, Raitanen J, Auvinen A, Isola J, Haapasalo H. Female predominance in meningiomas cannot be explained by differences in progesterone, estrogen, or androgen receptor expression. J Neurooncol. 2006. 80: 1-7
13. Lee KL, Terris MK. Luteinizing hormone-releasing hormone agonists and meningioma: Atreatment dilemma. Urology. 2003. 62: 351-
14. Li Q, Coulson H, Klaassen Z, Sharma S, Ramalingam P, Moses KA. Emerging association between androgen deprivation therapy and male meningioma: Significant expression of luteinizing hormone-releasing hormone receptor in male meningioma. Prostate Cancer Prostatic Dis. 2013. 16: 387-90
15. Mancini I, Rotilio A, Coati I, Seracchioli R, Martelli V, Meriggiola MC. Presentation of a meningioma in a transwoman after nine years of cyproterone acetate and estradiol intake: Case report and literature review. Gynecol Endocrinol. 2017. p. 1-4
16. Matsuda Y, Kawamoto K, Kiya K, Kurisu K, Sugiyama K, Uozumi T. Antitumor effects of antiprogesterones on human meningioma cells in vitro and in vivo. J Neurosurg. 1994. 80: 527-34
17. Ter Wengel PV, Martin E, Gooren L, Den Heijer M, Peerdeman SM. Meningiomas in three male-to-female transgender subjects using oestrogens/progestogens and review of the literature. Andrologia. 2016. 48: 1130-7
18. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010. 99: 307-14






Alexandra
Posted June 9, 2018, 9:29 am
Cyproterone acetate (Androcur) should no longer be prescribed to transsexual women as studies have, time and time again, shown a strong link between this drug and the onset of meningiomas, even at low dosages (10 mg daily). Even if rare, the few individuals harmed by it will never have their vision restored. Is it worth the risk? I don’t think so, especially considering there are other ways to achieve the results we seek in transsexual women…other, safer anti-androgens and taking estrogen alone parenterally to reduce T, as is observed in men with advanced prostate carcinoma and appears to be quite safe too.