- Department of Neurosurgery, University of Puerto Rico, San Juan, United States.
Correspondence Address:
Orlando De Jesus, Department of Neurosurgery, University of Puerto Rico, San Juan, United States.
DOI:10.25259/SNI_964_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Orlando De Jesus. Multiple myeloma extramedullary relapse at the sellar and suprasellar region after autologous stem cell transplantation. 12-Jan-2024;15:13
How to cite this URL: Orlando De Jesus. Multiple myeloma extramedullary relapse at the sellar and suprasellar region after autologous stem cell transplantation. 12-Jan-2024;15:13. Available from: https://surgicalneurologyint.com/surgicalint-articles/multiple-myeloma-extramedullary-relapse-at-the-sellar-and-suprasellar-region-after-autologous-stem-cell-transplantation/
Abstract
Background: The effectiveness of autologous stem cell transplantation (ASCT) in preventing the development of central nervous system (CNS) plasmacytomas in multiple myeloma (MM) patients is not well understood. An ASCT patient who developed CNS extramedullary (EM) lesions is presented. The literature was reviewed for similar cases in which the transplant did not prevent the development of CNS lesions.
Case Description: A 42-year-old female was evaluated after complaining of a sudden severe headache and complete vision loss. Two years before, she was diagnosed with MM and treated with systemic chemotherapy and an ASCT. The patient was in remission; however, a new brain magnetic resonance imaging showed a sellar and suprasellar mass. Additional smaller lesions were identified at the parietal convexity and the splenium. Due to the history of MM and evidence of multiple intracranial lesions, it was suspected that the lesions were secondary to EM disseminated disease. Due to the sudden loss of vision, the patient underwent a right frontotemporal craniotomy with subtotal sellar/suprasellar tumor resection to decompress the optic nerves. Histopathological examination of the lesion confirmed an immunoglobulin A (IgA) EM sellar and suprasellar plasmacytoma.
Conclusion: In the majority of MM patients with CNS involvement, ASCT did not prevent the development of EM sellar plasmacytomas. The IgA subtype is associated with more aggressive disease biology for CNS relapses.
Keywords: Extramedullary, Multiple myeloma, Plasmacytoma, Relapse, Sella, Stem cell, Suprasellar, Transplantation
INTRODUCTION
Multiple myeloma (MM) is a plasma cell proliferative disorder displaying an abnormal increase of monoclonal immunoglobulins. The overall 5-year survival rate ranges from 40% to 82%.[
Central nervous system (CNS) involvement of MM is rare, occurring in 0.7–1% of the patients.[
CASE REPORT
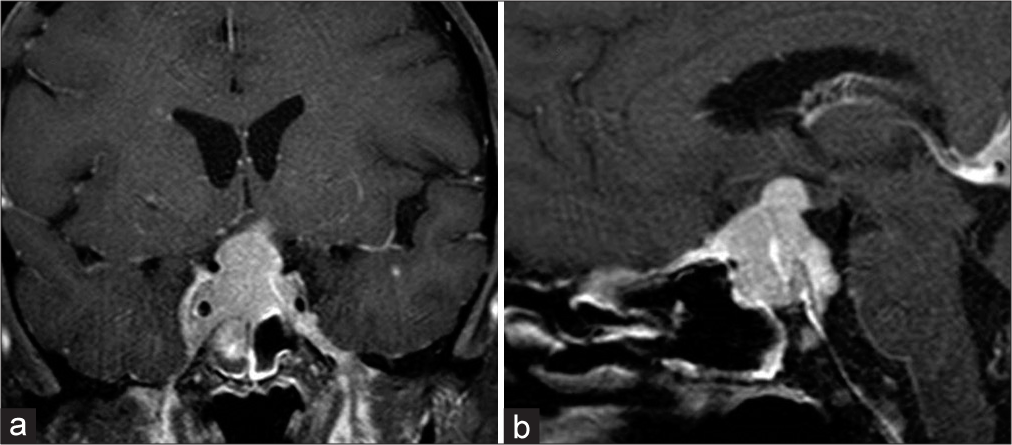
A 42-year-old female was evaluated at the emergency department (ED) after experiencing a severe headache and sudden complete vision loss. Two years before, she was diagnosed with MM and treated with systemic chemotherapy and an ASCT. She was in remission; however, eight months before her current evaluation, a right intracranial occipital dural base lesion was identified, for which she received fractionated radiotherapy. Three months later, brain magnetic resonance imaging (MRI) showed a significant reduction in the lesion size. During the two months before her evaluation at the ED, she noticed a slow but progressive visual acuity loss; however, she did not seek medical attention until she developed complete vision loss. A new brain MRI at the ED demonstrated a sellar and suprasellar mass measuring 1.9 cm anteroposterior × 2.1 cm transverse × 3.2 cm craniocaudal, showing avid contrast enhancement causing significant compression on the optic chiasm superiorly [
On physical examination, the patient had bilateral optic nerve swelling with no light perception. Bilateral six-nerve paresis was present. Motor and sensory examinations were normal. Due to the history of MM and evidence of multiple intracranial lesions, it was suspected that the lesions were secondary to her disseminated disease. Due to the sudden loss of vision, the patient underwent a right frontotemporal craniotomy with subtotal sellar/suprasellar tumor resection to decompress the optic nerves. The histopathological examination of the lesion was compatible with an immunoglobulin A (IgA) plasma cell neoplasia, CD19 positive, and negative for kappa or lambda light chain. She died three months later from her disseminated disease without receiving additional chemotherapy or radiotherapy.
DISCUSSION
Plasmacytomas are tumors that should be considered in the differential diagnosis for lesions involving the sella even in the absence of known MM, especially when cranial nerve paresis is present.[
Relapse of MM after ASCT usually presents with a recurrence of plasma cells in the marrow.[
Alegre et al. reported that 52% of MM patients relapsed or progressed after ASCT.[
CNS involvement following ASCT has been described in very few MM patients. Isolated CNS relapse after ASCT is extremely rare, with only 14 cases reported.[
This report highlighted an MM patient with an EM lesion at the sellar/suprasellar area that developed two years after receiving an ASCT. After reviewing the literature, four case reports of sellar/suprasellar plasma cell tumors were identified in which the patient received a stem cell transplant during the disease process.[
CONCLUSION
In the majority of patients with CNS MM involvement, ASCT did not prevent the development of EM sellar plasmacytomas. The IgA subtype is associated with more aggressive disease biology for CNS relapses.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdallah AO, Atrash S, Shahid Z, Jameel M, Grazziutti M, Apewokin S. Patterns of central nervous system involvement in relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2014. 14: 211-4
2. Alegre A, Granda A, Martínez-Chamorro C, Díaz-Mediavilla J, Martínez R, García-Laraña J. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: Clinical results of 280 cases from the Spanish Registry. Haematologica. 2002. 87: 609-14
3. Annibali O, Nobile C, Greco R, Cellini F, Quattrocchi CC, Tirindelli MC. The combination of topotecan, temozolomide, and dexamethasone is associated with radiotherapy as a treatment of central nervous system myeloma relapse. Int J Hematol. 2009. 89: 513-6
4. Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996. 335: 91-7
5. Bergantim R, Bastos J, Soares MJ, Carvalho B, Soares P, Marques C. Aggressive central nervous system relapse after autologous stem cell transplant in multiple myeloma: Case reports and literature review. Case Rep Hematol. 2020. 2020: 8563098
6. Bove V, Garrido D, Riva E. Young age and autologous stem cell transplantation are associated with improved survival in newly diagnosed multiple myeloma. Hematol Transfus Cell Ther. 2021. 43: 295-302
7. Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003. 348: 1875-83
8. Chu TH, Jung SH, Kim K, Lee JH, Mun JC, Bang SM. Relapse with plasmacytoma after upfront autologous stem cell transplantation in multiple myeloma. Ann Hematol. 2022. 101: 1217-26
9. Desikan R, Barlogie B, Sawyer J, Ayers D, Tricot G, Badros A. Results of high-dose therapy for 1000 patients with multiple myeloma: Durable complete remissions and superior survival in the absence of chromosome 13 abnormalities. Blood. 2000. 95: 4008-10
10. DiDomenico J, Ampie L, Choy W, Lamano JB, Oyon DE, Kesavabhotla K. Sellar plasmacytomas masquerading as pituitary adenomas: A systematic review. J Clin Neurosci. 2018. 50: 20-3
11. Erkus M, Bolaman Z, Meteoglu I, Kadikoylu G. Localized extramedullary relapse after autologous hematopoietic stem cell transplantation in multiple myeloma. Saudi Med J. 2005. 26: 989-91
12. Facon T, Avet-Loiseau H, Guillerm G, Moreau P, Geneviève F, Zandecki M. Chromosome 13 abnormalities identified by FISH analysis and serum beta2-microglobulin produce a powerful myeloma staging system for patients receiving high-dose therapy. Blood. 2001. 97: 1566-71
13. Fukai J, Nohgawa M, Uematsu Y, Itakura T, Kamei I. Immunoglobulin D multiple myeloma involving the sella manifesting as oculomotor palsy: Case report. Neurosurgery. 2010. 67: E505-6
14. Hotta M, Ito T, Konishi A, Yoshimura H, Nakanishi T, Fujita S. Multiple myeloma with central nervous system relapse early after autologous stem cell transplantation: A case report and literature review. Intern Med. 2021. 60: 463-8
15. Jiang CZ, Lin QS, Wu XY, Wang CY, Kang DZ. Sellar solitary plasmacytoma progressing to multiple myeloma: A case report and literature review. Medicine (Baltimore). 2014. 93: e58
16. Jurczyszyn A, Grzasko N, Gozzetti A, Czepiel J, Cerase A, Hungria V. Central nervous system involvement by multiple myeloma: A multi-institutional retrospective study of 172 patients in daily clinical practice. Am J Hematol. 2016. 91: 575-80
17. Khan IS, Javalkar V, Thakur JD, Nanda A. Intrasellar plasmacytoma: An illustrative case and literature review. J Clin Neurosci. 2012. 19: 210-3
18. Lee J, Kulubya E, Pressman BD, Mamelak A, Bannykh S, Zada G. Sellar and clival plasmacytomas: Case series of 5 patients with systematic review of 65 published cases. Pituitary. 2017. 20: 381-92
19. Li J, Shen KN, Huang WR, Li LH, Chen H, Chen WM. Autologous stem cell transplant can overcome poor prognosis in patients with multiple myeloma with extramedullary plasmacytoma. Leuk Lymphoma. 2014. 55: 1687-90
20. Li X, Wang W, Zhang X, Liang Y. Multiple myeloma with isolated central nervous system relapse after autologous stem cell transplantation: A case report and review of the literature. Front Oncol. 2022. 12: 1027585
21. Liu J, Shen J, Liu D. Case reports: Central nervous system involvement in patients with newly diagnosed multiple myeloma. Front Neurol. 2023. 14: 1072490
22. Mittal A, Pushpam D, Kumar L. Isolated central nervous system relapse of multiple myeloma post autologous stem cell transplant-A rare presentation. Leuk Res Rep. 2020. 14: 100207
23. Nishimura KK, Barlogie B, van Rhee F, Zangari M, Walker BA, Rosenthal A. Long-term outcomes after autologous stem cell transplantation for multiple myeloma. Blood Adv. 2020. 4: 422-31
24. Paludo J, Painuly U, Kumar S, Gonsalves WI, Rajkumar V, Buadi F. Myelomatous involvement of the central nervous system. Clin Lymphoma Myeloma Leuk. 2016. 16: 644-54
25. Petersen SL, Wagner A, Gimsing P. Cerebral and meningeal multiple myeloma after autologous stem cell transplantation. A case report and review of the literature. Am J Hematol. 1999. 62: 228-33
26. Rezvani A, Shahriarirad R, Fallahi MJ, Zeighami A. Extramedullary relapse of immunoglobulin A-kappa myeloma manifesting as plasmacytoma of the pleura without bone marrow involvement and following autologous bone marrow transplant: A case report. J Med Case Rep. 2023. 17: 42
27. Rosiñol L, Beksac M, Zamagni E, Van de Donk NW, Anderson KC, Badros A. Expert review on soft-tissue plasmacytomas in multiple myeloma: Definition, disease assessment and treatment considerations. Br J Haematol. 2021. 194: 496-507
28. Sammartano V, Cerase A, Venanzi V, Mazzei MA, Vangone BE, Gentili F. Central nervous system myeloma and unusual extramedullary localizations: Real life practical guidance. Front Oncol. 2022. 12: 934240
29. Seftel MD, Maguire J, Voss N, Woodhurst WB, Dalal BI, Shepherd JD. Intra-cerebral relapse following prolonged remission after autologous stem cell transplantation for multiple myeloma. Leuk Lymphoma. 2002. 43: 2399-403
30. Sinnott BP, Hatipoglu B, Sarne DH. Intrasellar plasmacytoma presenting as a non-functional invasive pituitary macro-adenoma: Case report & literature review. Pituitary. 2006. 9: 65-72
31. Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: A longitudinal study on 1003 consecutive patients. Ann Oncol. 2010. 21: 325-30
32. Varga G, Mikala G, Gopcsa L, Csukly Z, Kollai S, Balázs G. Multiple myeloma of the central nervous system: 13 Cases and review of the literature. J Oncol. 2018. 2018: 3970169
33. Yue X, He D, Zheng G, Yang Y, Han X, Li Y. Analysis of high-risk extramedullary relapse factors in newly diagnosed MM patients. Cancers (Basel). 2022. 14: 6106
34. Zeiser R, Deschler B, Bertz H, Finke J, Engelhardt M. Extramedullary vs medullary relapse after autologous or allogeneic hematopoietic stem cell transplantation (HSCT) in multiple myeloma (MM) and its correlation to clinical outcome. Bone Marrow Transplant. 2004. 34: 1057-65