- Department of Neurosurgery, University of Toyama, Toyama, Japan.
- Department of Neurosurgery, Toyama Red Cross Hospital, Toyama, Japan.
Correspondence Address:
Satoshi Hori, Department of Neurosurgery, University of Toyama, Toyama, Japan.
DOI:10.25259/SNI_121_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Satoshi Hori1,2, Shoichi Nagai2, Kohtaro Tsumura2, Satoshi Kuroda1. Mutism due to a massive hematoma after rebleeding of an aneurysmal subarachnoid hemorrhage in the territory of the distal anterior cerebral artery. 04-Mar-2022;13:79
How to cite this URL: Satoshi Hori1,2, Shoichi Nagai2, Kohtaro Tsumura2, Satoshi Kuroda1. Mutism due to a massive hematoma after rebleeding of an aneurysmal subarachnoid hemorrhage in the territory of the distal anterior cerebral artery. 04-Mar-2022;13:79. Available from: https://surgicalneurologyint.com/surgicalint-articles/11421/
Abstract
Background: The mutism caused by hematoma after subarachnoid hemorrhage (SAH) is extremely rare, and the details of its clinical course have not been clarified.
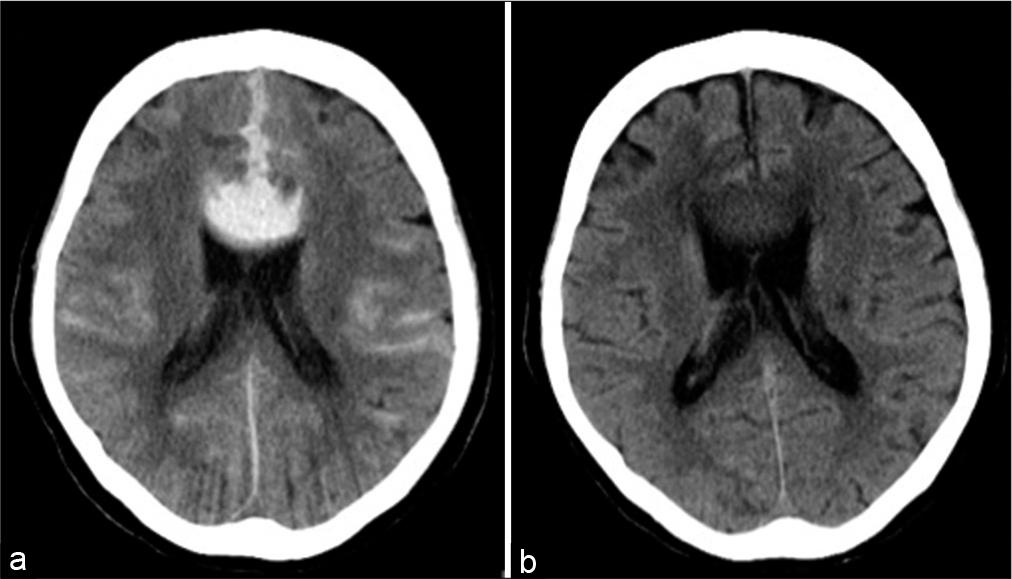
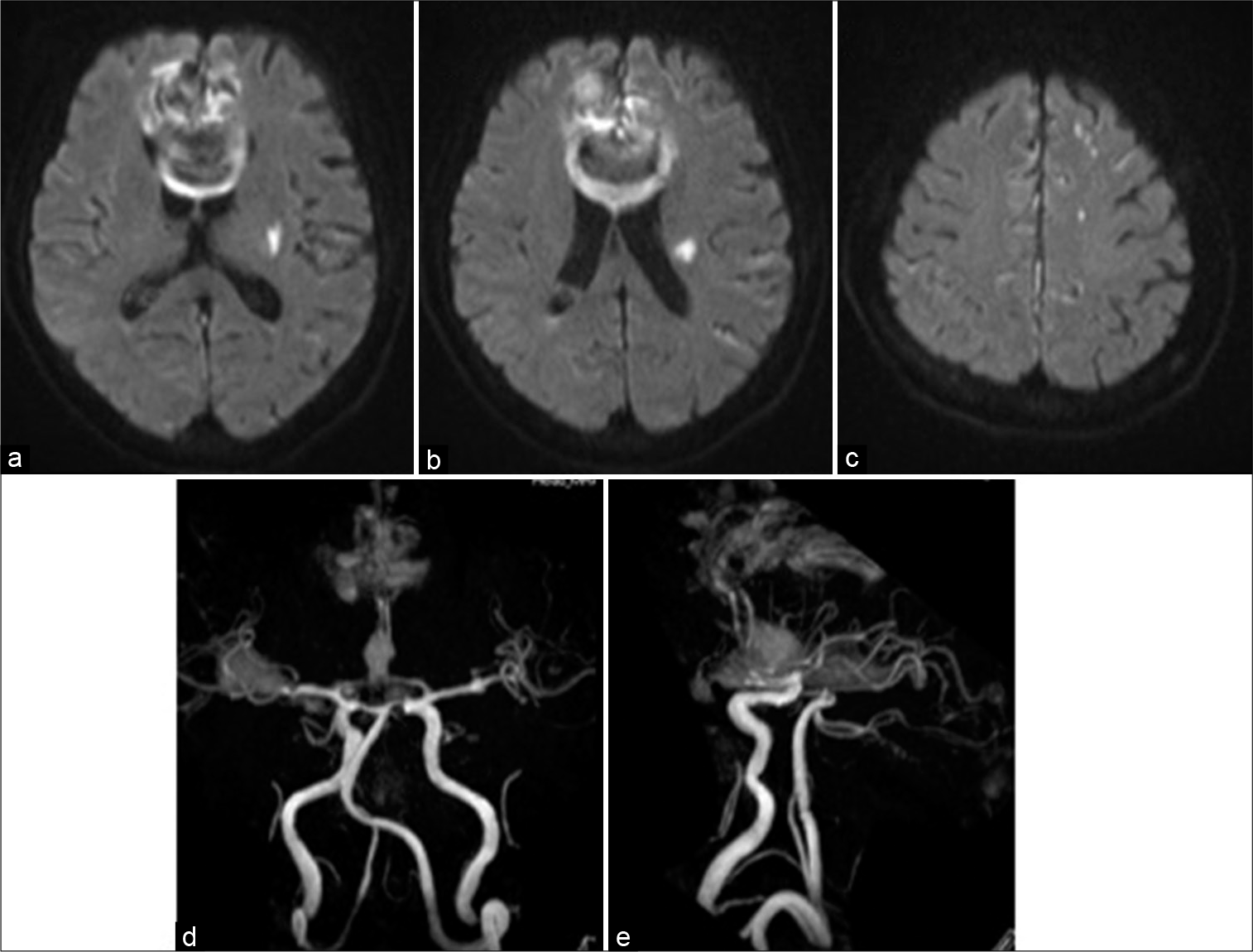
Case Description: A 75-year-old woman who presented with transient loss of consciousness and a subsequent severe headache was transferred to our hospital. She was diagnosed with the World Federation of Neurosurgical Societies Grade II SAH due to the rupture of an aneurysm at the A2–3 junction in the left anterior cerebral artery (ACA). Endovascular coil embolization was successfully performed; however, postoperative computed tomography (CT) confirmed a massive hematoma in the corpus callosum and expansion into the cingulate gyrus, which was suspected to be due to preoperative or intraoperative rebleeding. The patient remained completely mum, which was considered as mutism due to a hematoma in the ACA territory. The postoperative clinical course was favorable, and the patient had fully recovered speech fluency with the disappearance of hematoma on CT scan at 44 days after the occurrence of SAH.
Conclusion: This is a rare case of mutism caused by an interhemispheric hematoma due to rebleeding after SAH. No radical evacuation of the hematoma may be desirable for the improvement of mutism because additional structural damage to the ACA territory by surgical stress should be avoided.
Keywords: Distal anterior cerebral artery, Hematoma, Mutism, Subarachnoid hemorrhage
INTRODUCTION
The term mutism refers to a clinical state characterized by the complete or almost complete absence of speech that is not associated with other aphasic symptoms or deteriorated consciousness. The condition of mutism tends to be transient and is rarely observed in conscious neurological patients. The areas responsible for mutism include the supplementary motor area, corpus callosum, anterior cingulate gyrus, ventral lateral thalamus, periaqueductal gray region, and dentate nucleus.[
Mutism is normally observed in cases of head trauma, brain neoplasia, and ischemic stroke; however, it is rarely confirmed in hemorrhagic stroke. In contrast to the relatively high incidence of aneurysmal subarachnoid hemorrhage (SAH) in the territory of the anterior cerebral artery (ACA), mutism complicating vasospasm-related cerebral infarction is uncommon, much less due to the massive hematoma after SAH within the supplementary motor area, corpus callosum, and anterior cingulate gyrus.[
In this report, we describe a rare case of mutism due to a massive hematoma after rebleeding of aneurysmal SAH in the territory of the distal ACA and consider the optimal treatment strategy for SAH patients with this uncommon symptom.
CASE DESCRIPTION
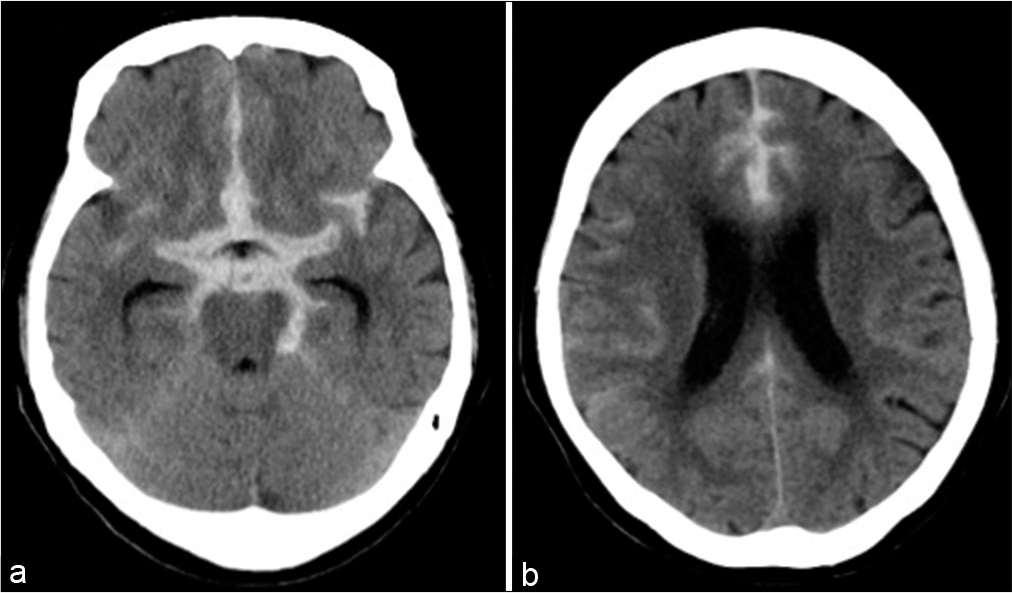
A 75-year-old woman presented with transient loss of consciousness and subsequent severe headache at home and was transferred to the emergency department of our hospital. She had a Glasgow Coma Scale score of 13/15 (E3V4M6) and no obvious focal neurological symptoms on admission. Head computed tomography (CT) revealed a Fisher Group 3 SAH uncomplicated with acute hydrocephalus [
Figure 2:
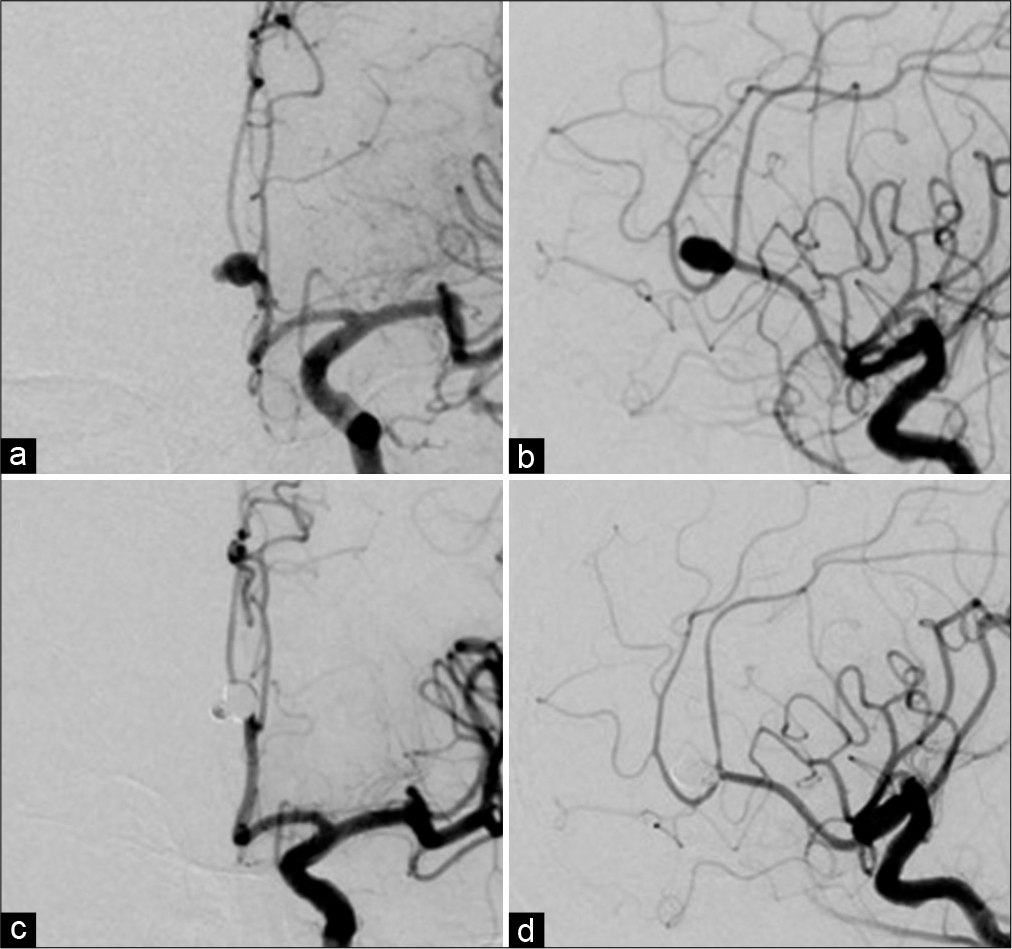
Digital subtraction angiography (a) anteroposterior (AP) projection, (b) lateral projection showing a saccular aneurysm at the A2–3 junction of the left anterior cerebral artery with a daughter sac. Endovascular coil embolization is executed successfully (c) AP projection, (d) lateral projection.
On day 1, endovascular coil embolization was successfully performed [
DISCUSSION
Mutism due to vascular disease is commonly caused by cerebral ischemia of the basilar artery, ACA territories, or the bilateral thalamic region. A literature review demonstrated that the most common cause of mutism in patients with SAH is secondary infarction in the distal ACA territory.[
To the best of our knowledge, three cases of mutism caused by hematoma due to SAH have been reported, which showed a massive interhemispheric subpial clot located dorsal to the corpus callosum splaying the cingulate gyri without the confirmation of secondary bilateral infarction in these territories.[
The prognosis of mutism due to hematoma after SAH has not yet been clarified. The case of existing permanent damage to the cingulate gyri is an unfavorable outcome compared to that of reversible compression. Choudhari et al. suggested that acute surgical or endovascular intervention might worsen the neurological state of patients who already had mutism and that the postponement of surgical treatment may be better for weeks following the onset. They believe that delayed intervention would maximize the chances of spontaneous neurological improvement.[
On the other hand, early intervention for aneurysmal SAH is desirable to avoid the danger of rebleeding with fatal consequences. In the previous three cases of mutism due to hematoma after SAH that underwent surgical intervention in the acute phase, one showed a slight improvement of symptoms after 3 months in spite of efforts to evacuate the clot,[
CONCLUSION
We described a rare case of mutism caused by an interhemispheric hematoma due to rebleeding after SAH. Acute surgical intervention for these patients could be performed safely, and radical evacuation of hematoma is not necessary for favorable outcomes of mutism. However, the etiology and prognosis of this uncommon neurological state are not fully understood; therefore, further investigations are needed to clarify this in the future.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Buge A, Escourolle R, Rancurel G, Poisson M. Akinetic mutism and bicingular softening. 3 anatomo-clinical cases. Rev Neurol (Paris). 1975. 131: 121-31
2. Burruss JW, Chacko RC. Episodically remitting akinetic mutism following subarachnoid hemorrhage. J Neuropsychiatry Clin Neurosci. 1999. 11: 100-2
3. Choudhari KA. Subarachnoid haemorrhage and akinetic mutism. Br J Neurosurg. 2004. 18: 253-8
4. Danilewicz B, Czepko R, Pyrich M, Betlej M, Stachura K, Zawiliński J. Immediate and late results of surgical treatment of hydrocephalus after subarachnoid hemorrhage. Przegl Lek. 1990. 47: 678-81
5. Nakasu Y, Isozumi T, Nioka H, Handa J. Mechanism of mutism following the transcallosal approach to the ventricles. Acta Neurochir (Wien). 1991. 110: 146-53
6. Sibille FX, Hantson P, Duprez T, van Pesch V, Giglioli S. Reversible akinetic mutism after aneurysmal subarachnoid haemorrhage in the territory of the anterior cerebral artery without permanent ischaemic damage to anterior cingulate gyri. Case Rep Neurol Med. 2016. 2016: 5193825
7. Tahta K, Cirak B, Pakdemirli E, Suzer T, Tahta F. Postoperative mutism after removal of an anterior falcine meningioma. J Clin Neurosci. 2007. 14: 793-6
8. Yanagawa Y, Itoh Y, Sakamoto T, Okada Y, Tokumaru AM. Post-traumatic mutism caused by corpus callosum injury diagnosed by fluid-attenuated inversion recovery on magnetic resonance imaging. J Trauma. 2005. 58: 631-3