- Department of Neurosurgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States.
Correspondence Address:
Ahmed Habib, Department of Neurosurgery, University of Pittsburgh, Pittsburgh, Pennsylvania, United States.
DOI:10.25259/SNI_469_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Habib, Nicolina Jovanovich, Meagan Hoppe, N.U. Farrukh Hameed, Lincoln Edwards, Pascal Zinn. Navigated 3D ultrasound-guided resection of high-grade gliomas: A case series and review. 12-Aug-2022;13:356
How to cite this URL: Ahmed Habib, Nicolina Jovanovich, Meagan Hoppe, N.U. Farrukh Hameed, Lincoln Edwards, Pascal Zinn. Navigated 3D ultrasound-guided resection of high-grade gliomas: A case series and review. 12-Aug-2022;13:356. Available from: https://surgicalneurologyint.com/surgicalint-articles/11789/
Abstract
Background: The crux in high-grade glioma surgery remains maximizing resection without affecting eloquent brain areas. Toward this, a myriad of adjunct tools and techniques has been employed to enhance surgical safety and efficacy. Despite intraoperative MRI and advanced neuronavigational techniques, as well as augmented reality, to date, the only true real-time visualization tool remains the ultrasound (US). Neuroultrasonography is a cost-efficient imaging modality that offers instant, real-time information about the changing anatomical landscape intraoperatively. Recent advances in technology now allow for the integration of intraoperative US with neuronavigation.
Case Description: In this report, we present the resection technique for three cases of high-grade gliomas (two glioblastomas and one anaplastic astrocytoma). The patient presented with a variable clinical spectrum. All three cases have been performed using the Brainlab® neuronavigation system (BrainLAB, Munich, Germany) and the bk5000 US Machine® (BK Medical, Analogic Corporation, Peabody, Massachusetts, USA).
Conclusion: Gross total resection was achieved in all three cases. The use of 3D navigated US was a reliable adjunct surgical tool in achieving favorable resection outcomes in these patients.
Keywords: 3D, High-grade, Navigated, Report, Ultrasound
INTRODUCTION
Gliomas are among the most devastating diseases known to mankind and currently, safe maximal surgical resection followed by adjuvant chemoradiation therapy is the standard of care.[
Preoperative imaging and neuronavigational systems are essential tools for routine surgical planning and resection of brain tumors.[
Intraoperative ultrasonography (IoUS) is a versatile alternative that has been used broadly across surgical disciplines, such as in breast, liver, and pancreatic operations.[
The current literature lacks sufficient evidence of the efficacy and the applicability of navigated 3D US in neurosurgical oncology. In this article, we aim to demonstrate and assess the value of navigated 3D US in resecting HGGs, with an emphasis on its supporting role in tumor mapping, preservation of key structures, locating residual tumor, and identifying possible hematoma accumulation during closure. Here, we report three consecutive HGG cases where integration of navigated 3D US in the surgical workflow aided in achieving maximal safe resection of tumors. We also discuss the literature examining the benefits and limitations of navigated 3D US compared to other neurosurgical imaging modalities, as well as examine its potential role in the future of neurosurgical oncology.
CASE REPORTS AND CLINICAL MATERIALS
Patients and 3D navigated US
Retrospective data collection was performed on three patients; (three females) with a mean age of 56 years (Range 32–68). All patients presented with diagnosed high-grade gliomas (two glioblastomas [GBMs] and one anaplastic astrocytoma). All three cases have been performed using the Brainlab® neuronavigation system (BrainLAB, Munich, Germany) and the bk5000 US Machine® (BK Medical, Analogic Corporation, Peabody, Massachusetts, USA). The operating room setup during US-guided resection of the three cases is shown in
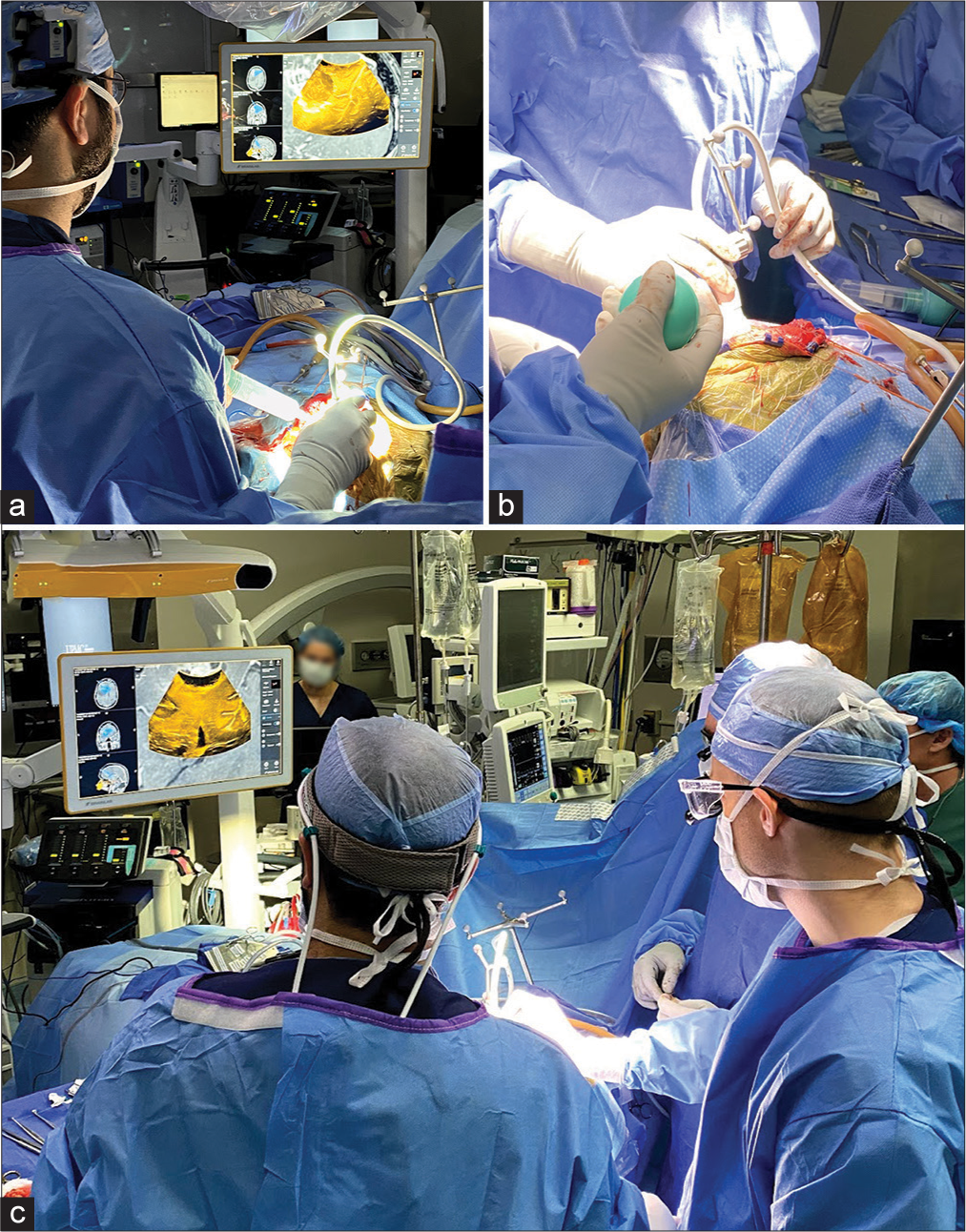
Figure 1:
A figure representation of the operating room setup during ultrasound-guided resection of high-grade gliomas. Note the digital integration of the bk5000 ultrasound device images with the conventional Brainlab navigation console. The Brainlab ultrasound navigation integration provides updated and accurate real-time overlay of ultrasound imaging on preoperative MRI/CT providing immediate and accurate information that accounts for brain shift during resection.
Patient 1
The patient is a 68-year-old Caucasian female who presented with worsening gait instability and recurrent falls. The patient was diagnosed with GBM WHO Grade IV and was initially treated at an outside facility with subtotal resection, concomitant radiation, and three cycles of temozolomide. Six months later, the patient presented to our facility with personality changes and mild expressive aphasia, increased nodular contrast enhancement inside, and extending from the prior resection cavity, as well as increasing enhancement tracking through the genu of the corpus callosum with marked surrounding vasogenic edema extending through the left frontal lobe [
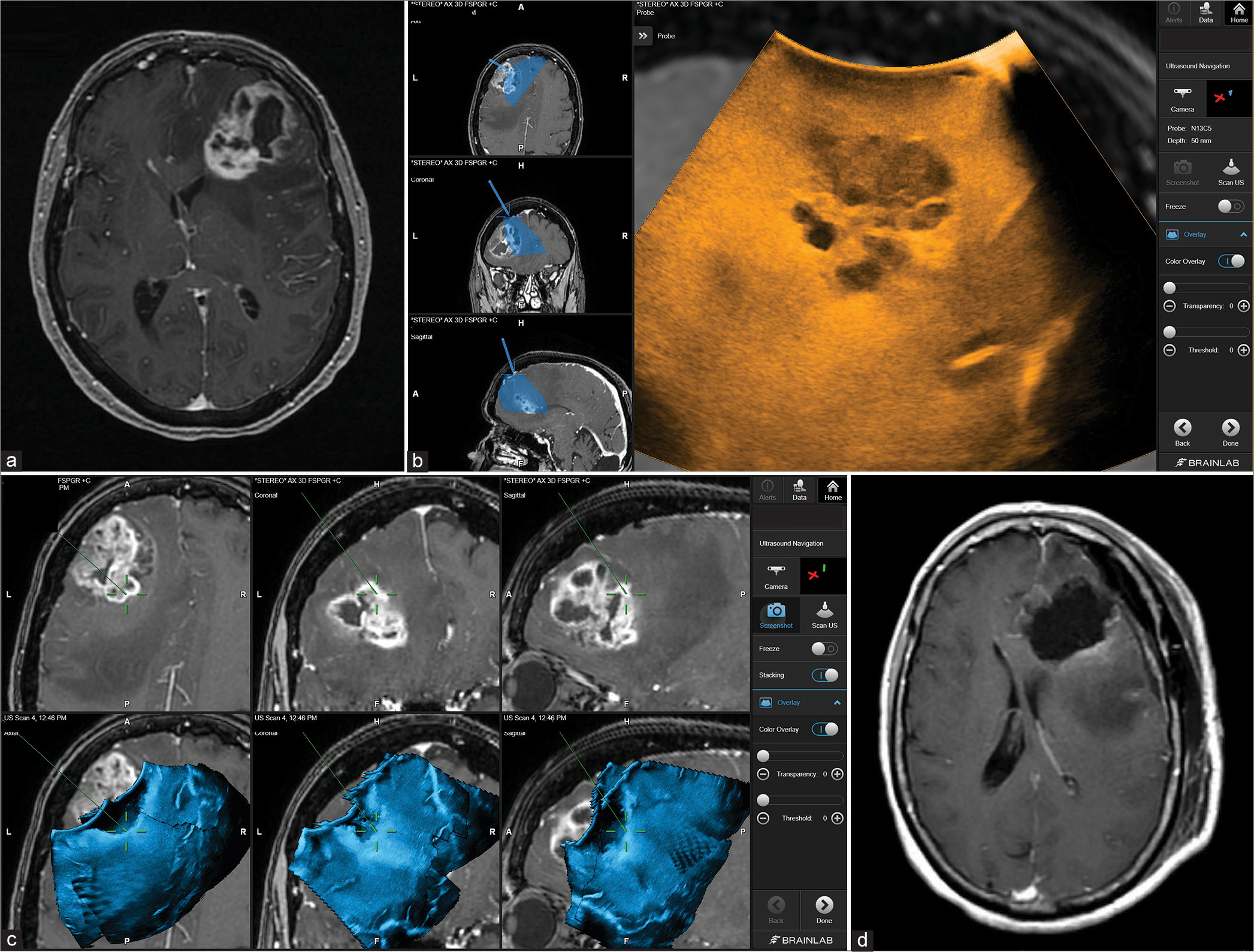
Figure 2:
Patient 1. Preoperative axial MRI with contrast showing the extent of tumor involvement in the left frontal lobe (a). Intraoperative Images showing the integration of real-time ultrasound images with the Brainlab navigation system (b). Using real-time ultrasound to safely and efficiently determine the extent of resection of the tumor bed and effectively accounting for possible brain shift towards the end of surgery (c). Postoperative axial MRI with contrast showing complete tumor resection (d).
Patient 2
The patient is a 68-year-old female with a medical history of sarcoidosis presented with a 1-month history of mental status change, right-sided headaches, and difficulty finding words that have significantly worsened over the past 2 weeks before admission. MRI demonstrated an ill-defined heterogeneously enhancing 8.7 cm mass in the left parietal and temporal lobes with mass effect [
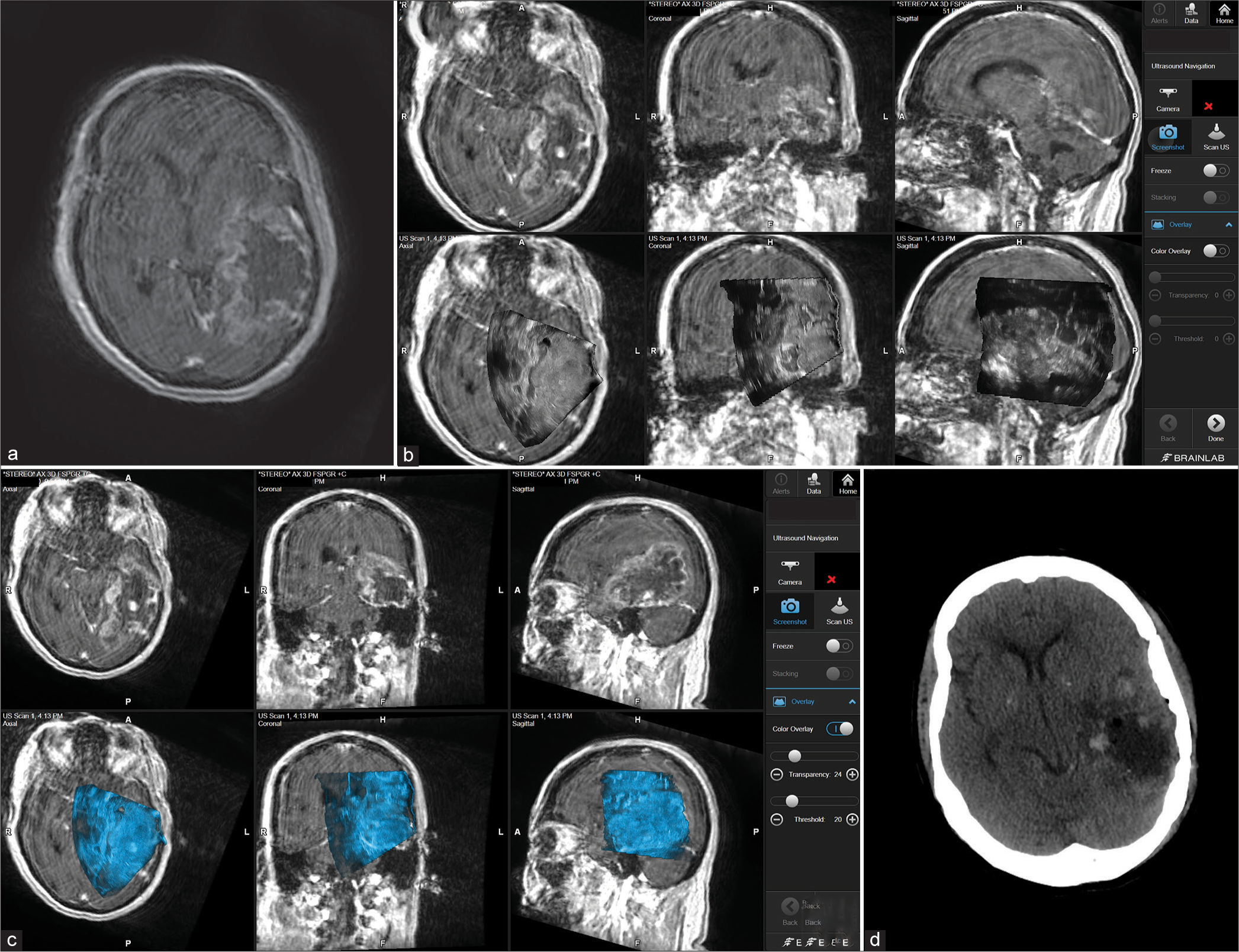
Figure 3:
Patient 2. Preoperative axial MRI with contrast showing massive temporoparietal involvement with high-grade glioma (a). Intraoperative images showing the integration of real-time ultrasound images with the Brainlab navigation system during the course of surgery (b and c). Postoperative axial CT scan showing complete tumor resection (d).
Patient 3
The patient is a 32-year-old female with no significant medical history who presented to our institution from an outside hospital after an incidental brain mass was found 1 month before presentation. On examination, the neurological examination was normal. MRI demonstrated an anterior frontal lobe mass which was predominantly extra-axial with T2 sequence hyperintensity and CSF cleft sign on sagittal T1-weighted images. The mass showed vague contrast-enhancement and minimal vasogenic edema. On FLAIR images, the mass was hyperintensity and measured 5.7 × 4.4 cm [
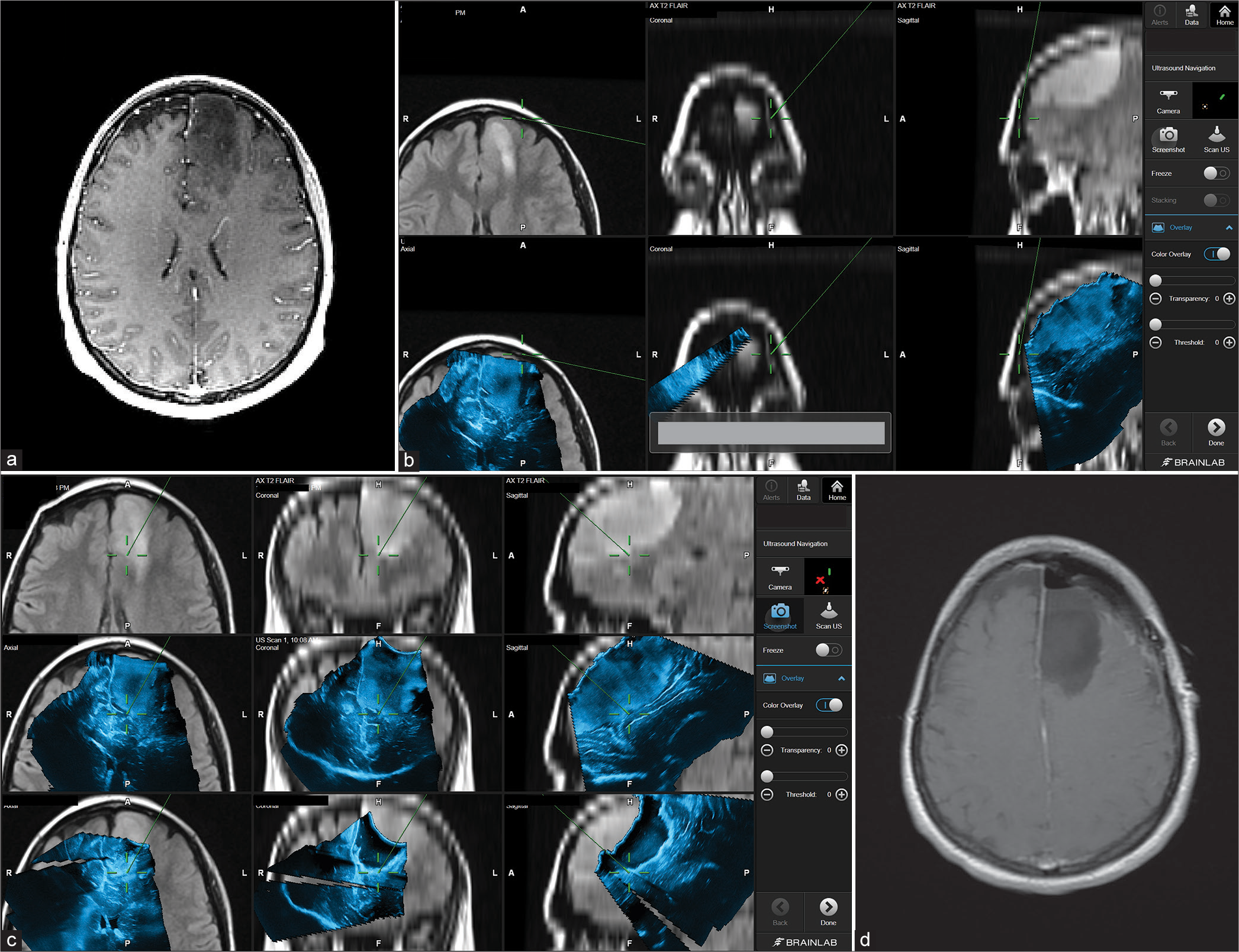
Figure 4:
Patient 3. Preoperative axial MRI with contrast showing left frontal lobe involvement with high-grade glioma (Anaplastic astrocytoma) (a). Intraoperative images showing the integration of real-time ultrasound images with the Brainlab navigation system during the course of surgery (b and c). In this case, it is apparent how navigated ultrasound can also help in planning the surgical course at the beginning of the surgery (b) and the role of assuring tumor bed safe margins toward the end of the resection (c). Postoperative axial MRI scan showing complete tumor resection (d).
REVIEW OF THE LITERATURE AND DISCUSSION
The application of US in neurosurgery dates back to the 1950s when French et al. used ultrasonics to locate subcortical neoplasms in autopsied brains.[
Over the past 20 years, neuronavigation has become an incredibly useful tool in providing guidance during neurosurgical procedures. The brain is a geometric volume; therefore, it can be divided into Cartesian planes, and any space within those planes can be defined by a set of coordinates. Neuronavigational systems take advantage of this by utilizing the tip of a probe to define a space within the brain in terms of these coordinates and then matching those coordinates to a parallel coordinate system in a 3D image. This allows surgeons to recognize the specific location of the brain; they are operating on, in the context of the preoperative scans, and can guide resection plans with great spatial accuracy. It is especially helpful in procedures that involve deep structures of the brain with highly important neurovascular structures.[
The low cost, absence of radiation, real-time feedback, and minimal anatomical disruption make the use of IoUS in neurosurgical practice extremely appealing, especially with the minimal efficacy sacrifice that it has to offer.[
As navigated 3D US techniques continue to evolve, an increase in image definition is expected. While image fusion between preoperative MRIs and navigated 3D U/S has eliminated the issue surrounding the plane of insolation and interpretation of U/S images in comparison to other modalities, the issue of artifacts still remains.[
CONCLUSION
Surgical eradication of high-grade gliomas remains a challenge for neurosurgeons due to the complex anatomy and the invasive nature of such tumors. The introduction of novel neuronavigation modalities combined with advanced imaging techniques could facilitate achieving a maximal resection which, in turn, correlates with better survival rates for patients with high-grade gliomas.[
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016. 17: 1521-32
2. Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014. 16: 81-91
3. Bozinov O, Burkhardt JK, Fischer CM, Kockro RA, Bernays RL, Bertalanffy H. Advantages and limitations of intraoperative 3D ultrasound in neurosurgery. Technical note. Acta Neurochir Suppl. 2011. 109: 191-6
4. Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016. 2: 1460-9
5. Chandler WF, Knake JE, McGillicuddy JE, Lillehei KO, Silver TM. Intraoperative use of real-time ultrasonography in neurosurgery. J Neurosurg. 1982. 57: 157-63
6. Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol Imaging. 2010. 9: 291-310
7. Coenen VA, Krings T, Mayfrank L, Polin RS, Reinges MH, Thron A. Three-dimensional visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: First experiences and technical note. Neurosurgery. 2001. 49: 86-92
8. Doyle J, Khalafallah AM, Yang W, Sun Y, Bettegowda C, Mukherjee D. Association between extent of resection on survival in adult brainstem high-grade glioma patients. J Neurooncol. 2019. 145: 479-86
9. French LA, Wild JJ, Neal D. The experimental application of ultrasonics to the localization of brain tumors; preliminary report. J Neurosurg. 1951. 8: 198-203
10. Ganau M, Ligarotti GK, Apostolopoulos V. Real-time intraoperative ultrasound in brain surgery: Neuronavigation and use of contrast-enhanced image fusion. Quant Imaging Med Surg. 2019. 9: 350-8
11. Ganau M, Syrmos N, Martin AR, Jiang F, Fehlings MG. Intraoperative ultrasound in spine surgery: History, current applications, future developments. Quant Imaging Med Surg. 2018. 8: 261-7
12. Ganslandt O, Behari S, Gralla J, Fahlbusch R, Nimsky C. Neuronavigation: Concept, techniques and applications. Neurol India. 2002. 50: 244-55
13. Gerard IJ, Kersten-Oertel M, Petrecca K, Sirhan D, Hall JA, Collins DL. Brain shift in neuronavigation of brain tumors: A review. Med Image Anal. 2017. 35: 403-20
14. Gronningsaeter A, Kleven A, Ommedal S, Aarseth TE, Lie T, Lindseth F. SonoWand, an ultrasound-based neuronavigation system. Neurosurgery. 2000. 47: 1373-9
15. Hammoud MA, Ligon BL, elSouki R, Shi WM, Schomer DF, Sawaya R. Use of intraoperative ultrasound for localizing tumors and determining the extent of resection: A comparative study with magnetic resonance imaging. J Neurosurg. 1996. 84: 737-41
16. Hervey-Jumper SL, Berger MS. Role of surgical resection in low-and high-grade gliomas. Curr Treat Options Neurol. 2014. 16: 284
17. Iversen DH, Wein W, Lindseth F, Unsgård G, Reinertsen I. Automatic intraoperative correction of brain shift for accurate neuronavigation. World Neurosurg. 2018. 120: e1071-8
18. Jung TY, Jung S, Kim IY, Park SJ, Kang SS, Kim SH. Application of neuronavigation system to brain tumor surgery with clinical experience of 420 cases. Minim Invasive Neurosurg. 2006. 49: 210-5
19. Kearns KN, Sokolowski JD, Chadwell K, Chandler M, Kiernan T, Prada F. The role of contrast-enhanced ultrasound in neurosurgical disease. Neurosurg Focus. 2019. 47: E8
20. Khoshnevisan A, Allahabadi NS. Neuronavigation: Principles, clinical applications and potential pitfalls. Iran J Psychiatry. 2012. 7: 97-103
21. Lee JH, Park G, Hong GH, Choi J, Choi HS. Design considerations for targeted optical contrast agents. Quant Imaging Med Surg. 2012. 2: 266-73
22. Lindner D, Trantakis C, Renner C, Arnold S, Schmitgen A, Schneider J. Application of intraoperative 3D ultrasound during navigated tumor resection. Minim Invasive Neurosurg. 2006. 49: 197-202
23. Moiraghi A, Pallud J. Intraoperative ultrasound techniques for cerebral gliomas resection: Usefulness and pitfalls. Ann Transl Med. 2020. 8: 523
24. Moiyadi AV, Shetty P. Direct navigated 3D ultrasound for resection of brain tumors: A useful tool for intraoperative image guidance. Neurosurg Focus. 2016. 40: E5
25. Pan H, Wu N, Ding H, Ding Q, Dai J, Ling L. Intraoperative ultrasound guidance is associated with clear lumpectomy margins for breast cancer: A systematic review and meta-analysis. PLoS One. 2013. 8: e74028
26. Prada F, Mattei L, Del Bene M, Aiani L, Saini M, Casali C. Intraoperative cerebral glioma characterization with contrast enhanced ultrasound. Biomed Res Int. 2014. 2014: 484261
27. Quencer RM, Montalvo BM. Normal intraoperative spinal sonography. AJR Am J Roentgenol. 1984. 143: 1301-5
28. Reid MH. Ultrasonic visualization of a cervical cord cystic astrocytoma. AJR Am J Roentgenol. 1978. 131: 907-8
29. Saß B, Bopp M, Nimsky C, Carl B. Navigated 3-dimensional intraoperative ultrasound for spine surgery. World Neurosurg. 2019. 131: e155-69
30. Sastry R, Bi WL, Pieper S, Frisken S, Kapur T, Wells W. Applications of ultrasound in the resection of brain tumors. J Neuroimaging. 2017. 27: 5-15
31. Selbekk T, Jakola AS, Solheim O, Johansen TF, Lindseth F, Reinertsen I. Ultrasound imaging in neurosurgery: Approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir (Wien). 2013. 155: 973-80
32. Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011. 12: 997-1003
33. Shetty P, Yeole U, Singh V, Moiyadi A. Navigated ultrasound-based image guidance during resection of gliomas: Practical utility in intraoperative decision-making and outcomes. Neurosurg Focus. 2021. 50: E14
34. Šteňo A, Hollý V, Mendel P, Šteňová V, Petričková Ľ, Timárová G. Navigated 3D-ultrasound versus conventional neuronavigation during awake resections of eloquent low-grade gliomas: A comparative study at a single institution. Acta Neurochir (Wien). 2018. 160: 331-42
35. Sun MR, Brennan DD, Kruskal JB, Kane RA. Intraoperative ultrasonography of the pancreas. Radiographics. 2010. 30: 1935-53
36. Trevisi G, Barbone P, Treglia G, Mattoli MV, Mangiola A. Reliability of intraoperative ultrasound in detecting tumor residual after brain diffuse glioma surgery: A systematic review and meta-analysis. Neurosurg Rev. 2020. 43: 1221-33
37. Unsgaard G, Ommedal S, Muller T, Gronningsaeter A, Nagelhus Hernes TA. Neuronavigation by intraoperative three-dimensional ultrasound: Initial experience during brain tumor resection. Neurosurgery. 2002. 50: 804-12
38. Unsgaard G, Selbekk T, Muller TB, Ommedal S, Torp SH, Myhr G. Ability of navigated 3D ultrasound to delineate gliomas and metastases comparison of image interpretations with histopathology. Acta Neurochir (Wien). 2005. 147: 1259-69
39. Unsgård G, Lindseth F. 3D ultrasound-guided resection of low-grade gliomas: Principles and clinical examples. Neurosurg Focus. 2019. 47: E9
40. Villa A, Costantino G, Meli F, Odierna Contino A, Imperato A, Francaviglia N. Ultrasound-based real-time neuronavigated fluorescence-guided surgery for high-grade gliomas: Technical note and preliminary experience. Acta Neurochir (Wien). 2019. 161: 2595-605
41. Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014. 15: e395-403
42. Willems PW, van der Sprenkel JW, Tulleken CA, Viergever MA, Taphoorn MJ. Neuronavigation and surgery of intracerebral tumours. J Neurol. 2006. 253: 1123-36
43. Yeole U, Singh V, Mishra A, Shaikh S, Shetty P, Moiyadi A. Navigated intraoperative ultrasonography for brain tumors: A pictorial essay on the technique, its utility, and its benefits in neuro-oncology. Ultrasonography. 2020. 39: 394-406