- Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York City, New York, United States
- Global Neurosurgical Alliance, United States,
- College of Medicine, The University of Arizona College of Medicine - Tucson, Arizona, United States
- Department of Neurosurgery, University of Arizona, Tucson, Arizona, United States,
- Department of Neurosurgery, The University of Jordan School of Medicine, Amman, Jordan,
- Department of Medicine, Aga Khan University, Karachi, Pakistan,
- Department of Neurosurgery, University of Kufa College of Medicine, Kufa, Iraq,
- Department of Medicine, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom,
- College of Medicine, The University of Arizona College of Medicine - Phoenix, Phoenix, Arizona, United States.
Correspondence Address:
Albert Alan, Department of Neurosurgery, University of Arizona, Tucson, Arizona, United States.
DOI:10.25259/SNI_774_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Barbara Buccilli1,2, Albert Alan2,3,4, Baha’ Ghandi Aljeradat2,5, Akmal Shahzad2,6, Yasser F. Almealawy2,7, Nathan Simbarashe Chisvo2,8, Michelle Ennabe2,9, Martin Weinand3,4. Neuroprotection: Surgical approaches in traumatic brain injury. 26-Jan-2024;15:23
How to cite this URL: Barbara Buccilli1,2, Albert Alan2,3,4, Baha’ Ghandi Aljeradat2,5, Akmal Shahzad2,6, Yasser F. Almealawy2,7, Nathan Simbarashe Chisvo2,8, Michelle Ennabe2,9, Martin Weinand3,4. Neuroprotection: Surgical approaches in traumatic brain injury. 26-Jan-2024;15:23. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12724
Abstract
Background: This review is centered on the pivotal role of surgical interventions within the comprehensive management of traumatic brain injury (TBI). Surgical strategies are indispensable components of TBI care, encompassing primary injury management and the alleviation of secondary injury processes, including the handling of intracranial hemorrhages (ICHs), contusions, and mass lesions.
Methods: A systematic review was carried out by searching databases including PubMed, Embase, and Scopus. The inclusion criteria involved studies discussing surgical strategies for TBI, with a focus on primary injury management, ICHs, contusions, and mass lesions. More recent articles were prioritized, and data were synthesized to assess the impact of surgical interventions on TBI outcomes.
Results: The evolution of surgical technologies has heralded a transformation in TBI management. These advancements encompass minimally invasive procedures, neuroimaging-guided surgeries, and robotic-assisted techniques, all geared toward optimizing patient outcomes.
Conclusion: Surgical interventions within TBI care present unique challenges, such as timing considerations, patient selection criteria, and postoperative care. This review underscores the critical significance of multidisciplinary collaboration among neurosurgeons, neurologists, and critical care specialists. Such collaboration is essential to tailor surgical strategies to the individualized needs of patients. Moreover, the review highlights emerging trends in TBI surgery and underscores the ongoing imperative of research endeavors aimed at refining surgical protocols and ultimately enhancing patient outcomes.
Keywords: Cisternostomy, Decompressive craniectomy, Intracranial hemorrhages, Neuroimaging-guided surgeries, Traumatic brain injury
INTRODUCTION
Background and significance of TBI-related neuroprotection
Traumatic brain injury (TBI) is considered one of the leading causes of morbidity, disability, and mortality across all ages.[
Any trauma or injury to the brain triggers the activation of a local inflammatory response, primarily mediated by microglia.[
Primary injury is explained by the displacement of neural tissue and mechanical injury to it, including hemorrhages, vascular damage, contusions, changes in cerebral blood flow (CBF) and blood–brain barrier (BBB) permeability, and metabolic disturbances. Within minutes of the initial mechanical injury, complex biochemical reactions are triggered. These reactions extend for days, months, or even years after the initial injury, causing neuroinflammation, neurodegeneration, and neurological deficits.[
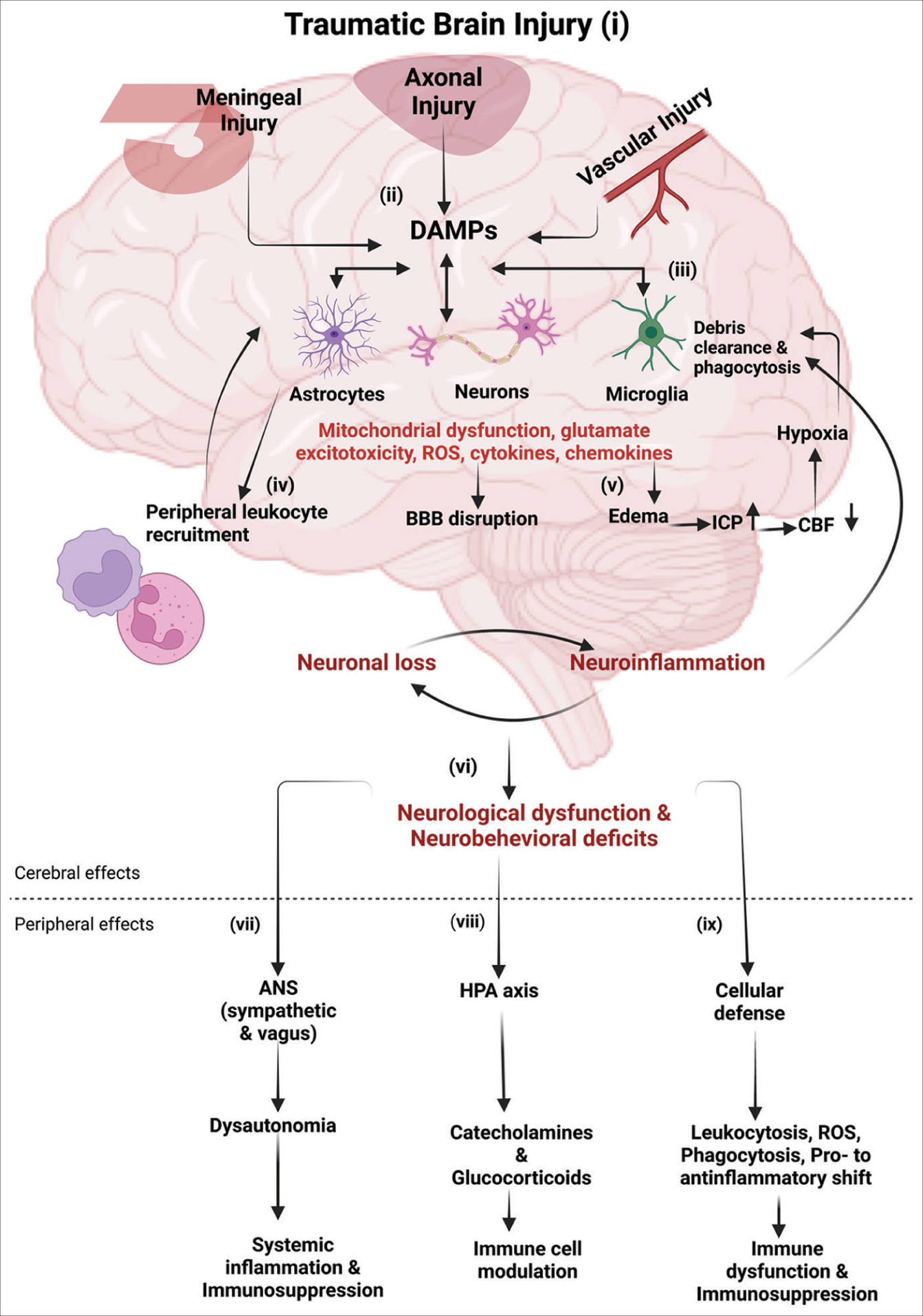
Figure 1:
Immune response following traumatic brain injury (TBI): (i-ii) following TBI, the primary mechanical injury can include meningeal contusion, axonal shearing, and cerebrovascular injury, culminating in meningeal and neuronal cell death, as well as microglial and astrocytic activation. (iii) Such neuronal injury and glial engagement generate chemokines, cytokines, and reactive oxygen species, along with the release of damage-associated molecular patterns (DAMPs), setting off an inflammatory response. (iv) In the presence of DAMPs, phagocytic microglia engage in debris clearance and synthesize neurotrophic agents. Sustained stimulation of these pathways induces subsequent injury through leukocyte recruitment, which initially aids in the removal of tissue debris. (v) Subsequently, it contributes to the progression of inflammation and disruption of the blood–brain barrier (BBB). The cytotoxic edema and compromised BBB integrity bring to an elevation of the intracranial pressure, leading to decreased cerebral blood flow, thereby intensifying hypoxia and disrupting the cerebral energy supply. Consequently, this cascade drives further neuronal depletion, propelling a self-perpetuating cycle of neuroinflammation and neurodegeneration. (vi) These progressive pathological modifications culminate in neurological dysfunction and deficits in motor, cognitive, and emotional functions. TBI also induces alterations in the autonomic nervous system (ANS), which monitors and regulates DAMPs, consequently eliciting both cerebral and peripheral immune responses. (vii) Activation of the sympathetic ANS culminates in the peripheral discharge of catecholamines (epinephrine and norepinephrine), which suppress the systemic immune responses of macrophages through the cholinergic anti-inflammatory pathway (CAO), thereby mitigating systemic inflammation. (viii) Furthermore, the release of catecholamines and glucocorticoids through the hypothalamic-pituitary-adrenal axis governs the functional behavior of systemic immune cells after TBI. (ix) The cellular immune response to traumatic brain injury involves an increase in leukocytosis and ROS generation, progresses through phagocytosis, and shifts from pro-inflammatory to anti-inflammatory states, potentially leading to immune dysfunction and immunosuppression. Abbreviations: ICP (increased intracranial pressure), CBF (cerebral blood flow), HPA (hypothalamic-pituitary-adrenal), ROS (reactive oxygen species). Image created with BioRender.com.
TBI is currently receiving a lot of public attention due to its social and financial costs.[
The incidence of TBI varies with age and sex, with males (and especially young men) being more likely to require a TBI-related hospital visit.[
The most common kind of TBI identified (70% of cases), mild TBI (mTBI) [
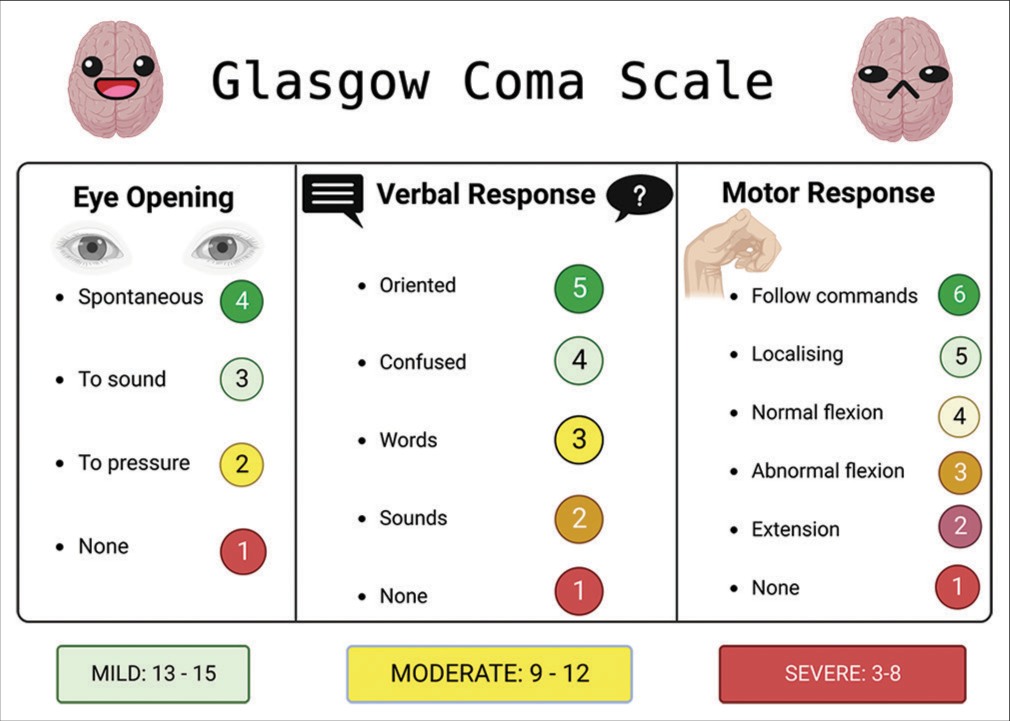
Figure 2:
This figure illustrates the Glasgow coma scale (GCS), a vital neurological assessment tool, as it pertains to traumatic brain injury (TBI). The GCS quantifies the patient’s level of consciousness based on eye, verbal, and motor responses, aiding clinicians in gauging TBI severity and guiding treatment decisions. Image created with BioRender.com.
The direct and immediate brain injury can cause two types of primary brain injury; focal and diffuse injuries.[
The primary injury often progresses to delayed and prolonged secondary injury. A number of factors contribute to secondary injury: Excitotoxicity, mitochondrial dysfunction, oxidative stress, lipid peroxidation, neuroinflammation, axon degeneration, and apoptotic cell death.[
Dysfunction of BBB occurs after 24 h of acute TBI, which allows infiltration of circulating neutrophils, monocytes, and lymphocytes into the injured brain parenchyma.[
Scope and objectives of the review
The scope of this review is to comprehensively explore and analyze surgical neuroprotection strategies employed in the context of TBI. Understanding the effectiveness of different interventions is essential to mitigate the impact of TBI and improve patient outcomes. By investigating the available literature and clinical trials, the review seeks to provide a comprehensive overview of the strengths and limitations of each strategy and their potential for translation into clinical practice. Ultimately, the objectives of this review are to inform healthcare practitioners, researchers, and policymakers about evidence-based neuroprotection strategies that hold promise in alleviating the consequences of TBI and fostering better patient recovery and quality of life.
METHODOLOGY
Inclusion and exclusion criteria for article selection
Inclusion and exclusion criteria play a crucial role in ensuring that the articles selected for this review are relevant and meet the objectives of the study. The following are the inclusion and exclusion criteria established for article selection: we included articles that directly address surgical neuroprotection strategies in the context of TBI and that focus on the prevention, reduction, or mitigation of brain injury after a TBI event. Articles published within the past 20 years were given priority to ensure that the review reflects current research and developments in the field. Both preclinical and clinical studies were included. This encompasses animal studies, in vitro experiments, as well as randomized controlled trials, cohort studies,case–control studies, and systematic reviews. Only articles written in English were included for ease of comprehension and analysis. Articles that are accessible through academic databases, online journals, and reputable sources were prioritized to ensure reliability and credibility.
Articles not directly related to neuroprotection in the context of TBI were excluded from the study. This includes studies focused solely on other brain disorders or general neurological conditions. Gray literature, conference abstracts, editorials, opinions, and non-peer-reviewed articles were excluded due to potential limitations in the rigor and credibility of the information presented. Articles not written in English were excluded, as translation resources may not be readily available and could introduce inaccuracies. In case of duplicate publications, only the most comprehensive and recent versions were included to avoid redundancy.
By adhering to these inclusion and exclusion criteria, the review aims to maintain a high standard of academic rigor, relevance, and reliability. The selected articles will contribute to a comprehensive and evidence-based analysis of surgical neuroprotection strategies in the context of TBI, enabling a meaningful synthesis of findings and implications for clinical practice and future research.
Search strategy and databases used
The following is an outline of the search strategy and the databases used.
We identified relevant keywords and phrases related to the topic, such as “traumatic brain injury,” “TBI,” “neuroprotection,” “neuroprotective agents,” “interventions,” “clinical trials,” and “brain injury outcome.” We then combined the identified keywords using Boolean operators (AND, OR) to create effective search strings. For example, (traumatic brain injury OR TBI) AND (neuroprotection OR neuroprotective agents), (neuroprotection OR neuroprotective interventions) AND (brain injury outcome OR clinical trials), (TBI, Neuroprotection, Outcomes evaluation, biomarkers, Imaging techniques, Challenges, “Brain injuries, traumatic”[Mesh], “Neuroprotection”[Mesh], “Outcome assessment, health care”[Mesh], “Biomarkers”[Mesh], and “Diagnostic Imaging”[Mesh]). We included synonyms, alternate spellings, and related terms to capture a broader range of relevant articles.
The databases included in our literature search are PubMed/MEDLINE, Embase, and Scopus. To ensure a thorough search, the reference lists of relevant review articles and included studies will be manually checked for potentially relevant articles that may not have appeared in the initial database search.
By employing this comprehensive search strategy and using reputable databases, the review aims to gather a diverse and extensive collection of literature on neuroprotection strategies in TBI, enabling a robust analysis and synthesis of the available evidence.
SURGICAL APPROACHES
Overview of surgical interventions for TBI neuroprotection
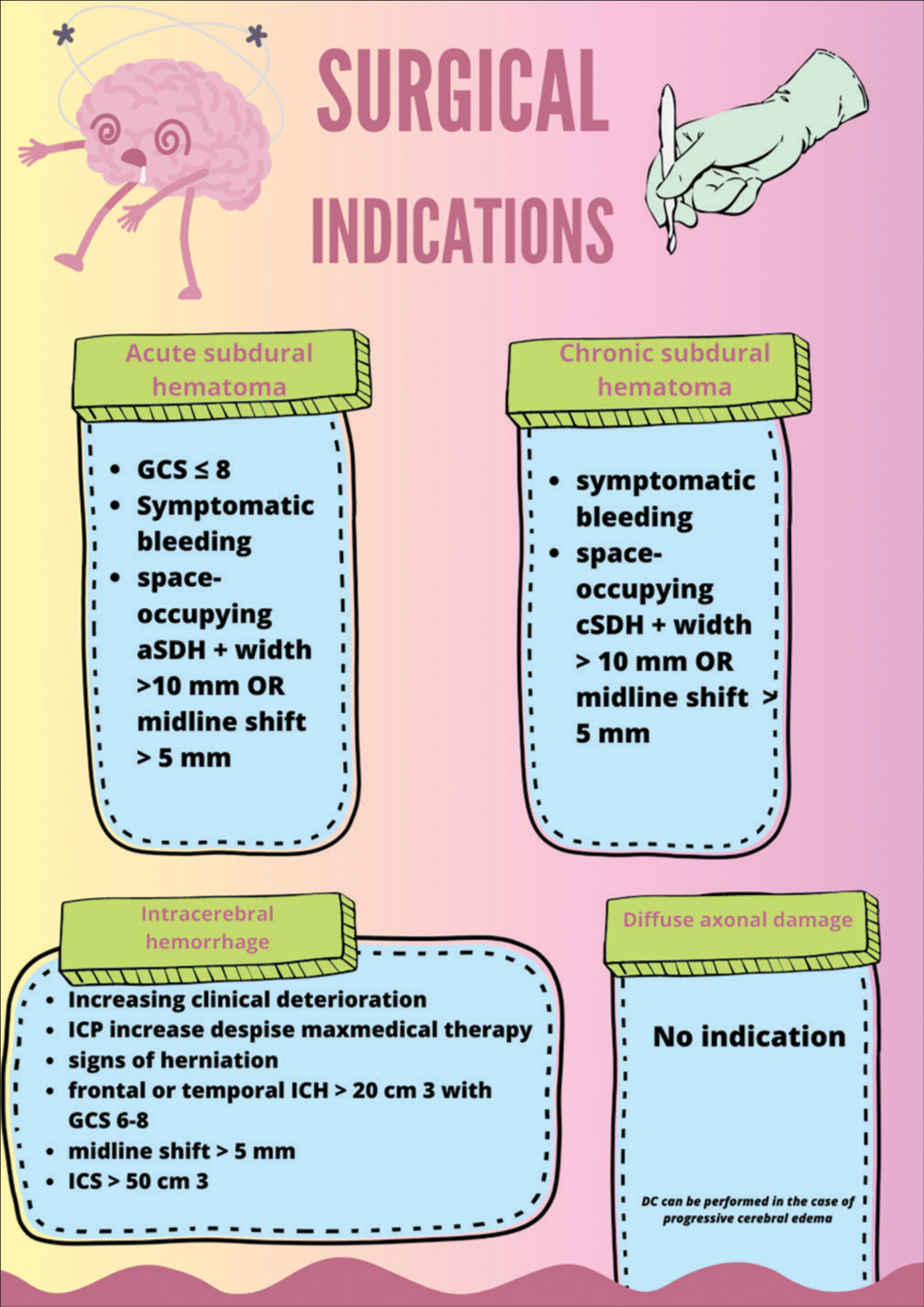
The surgical indications in TBI are broad [
Figure 3:
This figure delineates the criteria for surgical intervention across common traumatic brain injury scenarios. Specific clinical and radiological criteria guide the decision to opt for surgery.[
Regarding subdural hematomas (SDHs), those exceeding 1 cm, accompanied by midline shifts surpassing 5 mm, a GCS score below 8 with rapid deterioration, or intracranial pressure (ICP) below 20 mm Hg, should all factor into the decision-making process concerning potential evacuation.[
Decompression must be taken into account for individuals with parenchymal lesions who have progressive neurologic decline, mass effect, refractory intracranial hemorrhage (ICH), GCS scores of 6–8, frontal or temporal contusions >20 cm3, midline shift of a minimum of 5 mm, and/or compression of cisterns. This is particularly true if the patients also have lesion volumes >50 cm3.[
Decompressive craniectomy (DC) is a procedure that involves removing a significant section of the skull vault to lower ICP and the negative outcomes that it might lead to.[
In a patient with considerable concomitant underlying cerebral edema, isolated evacuation of the SDH without interim excision of the bone flap might lead to an additional decline in the patient’s conditions following the original operation. Nevertheless, it might be appropriate to perform a craniotomy, with hematoma evacuation and duraplasty, when the underlying brain damage is mild, and the hematoma itself mostly brings on the mass effect.[
Acute SDH begins to liquefy after a few days, making less invasive surgical evacuation possible through a bedside subdural bolt evacuating system. A little cut is made over the SDH, a burr hole is created with a portable twist drill, the dura is opened, and the metal bolt is inserted into the burr hole. The bolt is then secured with a tube that is coupled to a suction mechanism. The main disadvantage is that, when there are many subdural loculations and septations, only the pocket the bolt is covering can be emptied of blood. Surgical intervention may be used to treat a mixed-density SDH that is symptomatic.[
In cases of severe TBI, opening cisterns might reduce swelling and the need for decompressive hemicraniectomies through a backshift of cerebrospinal fluid (CSF) through the Virchow-Robin spaces.[
Evidence from animal models and preclinical studies
Fluid percussion injury (recently updated to a later version, which not only causes localized cortical contusions but also conveys the traumatic damage to subcortical structures, including the thalamus and hippocampi), control cortical impact, weight drop impact acceleration injury, and blast injury model are animal models of TBI which have been regularly employed try to mimic TBI for research purposes.[
Animal models of TBI have been used to study the effect of DC on the development of brain edema and subsequent damage following TBI, although the literature is controversial. According to some authors, early craniectomy can prevent later brain injury and markedly decrease cerebral edema accumulation.[
Clinical trials and surgical outcomes
The DECRA (DC in Diffuse TBI) study, which provided preventive DC (within 72 h of TBI) in patients with diffuse (on computed tomography [CT]) or severe (on GCS score) TBI, investigated the utility of secondary DC in refractory increased ICP after severe TBI.[
A subsequent multinational prospective randomized controlled trial study called randomized evaluation of surgery with craniectomy for uncontrollable elevation of ICP compared medical management alone with medical management together with DC as a treatment for TBI patients who had severe, persistent, and unresponsive intracranial hypertension.[
EVALUATING NEUROPROTECTION OUTCOMES
TBI remains a significant public health concern, and the identification of suitable outcome measures which are reliable and reflective of the full picture of the patient’s condition is extremely important.
The use of biomarkers in neuroprotection research
Biomarkers have emerged as critical tools in advancing our understanding of neuroprotection after TBI and in optimizing therapeutic approaches. A novel approach introduces that bioactive nanofibrous dural substitutes, fabricated using polycaprolactone nanofibers encapsulated with hyaluronic acid methacryloyl and insulin-like growth factor 1 (IGF-1), offer controlled release of IGF-1.[
A recent study pioneers the use of acute treatment with a TrkB agonist to confer neuroprotection and preserve myelin integrity. By leveraging spectral confocal reflectance microscopy, the study unveils the subtle yet crucial changes occurring at the cellular level.[
Expanding on this, another project highlights the potential of neuronal CD200 as a biomarker for predicting stroke outcomes and tailoring neuroprotective interventions by diving into the role of neuronal CD200 signaling in the acute phase of ischemic stroke.[
Research also explores the inhibition of high mobility group box 1 (HMGB1) as a strategy to modulate microglia/macrophage polarization after TBI.[
Another study focuses on the effectiveness of the NADPH oxidase 2 (NOX2) inhibitor GSK2795039 in providing neuroprotection. NOX2 is implicated in oxidative stress, a prominent contributor to TBI pathology.[
A similar study showcases the remarkable potential of gene therapy for neuroprotection.[
Collectively, these studies emphasize the pivotal role of biomarkers in neuroprotection research. From utilizing bioactive nanofibrous substitutes to modulating CD200 signaling, inhibiting HMGB1, targeting NOX2, and harnessing gene therapy, biomarkers serve as guiding beacons, illuminating the path toward effective neuroprotection strategies. By unraveling the molecular intricacies of TBI and its aftermath, researchers are poised to revolutionize how we approach and combat traumatic brain injuries.
The use of imaging modalities in neuroprotection research
Neuroprotection research is undergoing a paradigm shift with the integration of advanced imaging modalities. These cutting-edge techniques provide insights into the structural and functional changes that unfold in the brain following TBI.
Recent findings showed how imaging bridges the gap between TBI and Alzheimer’s disease.[
The utilization of advanced imaging techniques for assessing TBI has significant implications for diagnosis, understanding injury mechanisms, and predicting patient outcomes. CT plays a pivotal role in the initial evaluation by swiftly diagnosing brain injuries, identifying fractures, and guiding surgical decisions. While excelling at detecting focal injuries such as extradural and SDHs, CT has limitations in spotting subtle injuries such as traumatic axonal injury (TAI) and entails ionizing radiation exposure. CT-based scoring systems are explored for predicting TBI outcomes and mortality. Magnetic resonance imaging (MRI) emerges as a sensitive tool for detecting diffuse brain injuries, particularly TAI. Various MRI sequences, including fluid-attenuated inversion recovery (FLAIR) and susceptibility-weighted imaging (SWI), enhance TAI detection. Despite the usefulness of CT-based models for predicting major outcomes, MRI holds promise in identifying subtle neurological changes with functional significance. Advanced neuroimaging techniques offer insights into complex facets of TBI. Diffusion-weighted imaging (DWI) captures water movement, indicating constrained or free diffusion. Acute ischemia results in elevated signal intensity on DWI images, while regions with vasogenic edema appear illuminated on apparent diffusion coefficient maps. Diffusion tensor imaging provides insights into tissue damage, particularly in white matter. Standard MRI sequences (T1, T2, FLAIR, and SWI) excel in TBI diagnosis but come with limitations, requiring specialized equipment and lengthier scan times. Physiological imaging techniques shed light on TBI progression. Perfusion CT generates maps of CBF, aiding tissue viability evaluation. Positron emission tomography visualizes brain activities, revealing insights into TBI pathophysiology. Magnetic resonance spectroscopy (MRS) quantifies brain molecules, with changes indicating neuronal loss or metabolic dysfunction. Proton MRS predicts unfavorable outcomes, especially in specific brain regions. Phosphorous MRS offers insights into cerebral metabolic function. Chronic TBI analysis using advanced imaging methods uncovers reductions in brain volume in regions like the hippocampus. Resting and task-oriented functional MRI (fMRI) elucidate network changes and adaptation, highlighting cognitive deficits and reorganization. fMRI even challenges prior assumptions about patient responsiveness, reshaping recovery assessment post-brain injury.[
CHALLENGES
Neuroprotection research for TBI stands at the crossroads of scientific discovery and clinical translation, yet navigating this complex terrain is rife with challenges. Research sheds light on the intricacies of clinical trials focused on neuroprotective interventions.[
In the realm of age-related variations, a recent work confronts the complex challenge of translating neuroprotective interventions across different age groups.[
The longitudinal aspect of neurodegenerative diseases takes center stage, particularly when dealing with the intricacies of investigating gradual-progressing conditions. This becomes especially pertinent in the context of gene therapy for neuroprotection, where the necessity for enduring evaluations becomes evident. The formidable challenge of ensuring continuous interventions and evaluations over extended periods is highlighted, thus unveiling the intricate process of converting promising research into concrete clinical outcomes.[
CLINICAL IMPLICATIONS
Translating neuroprotection strategies to clinical practice
Enhancing TBI care requires an important transition from experimental neuroprotection techniques to practical applications in clinical settings. To enable the smooth incorporation of these strategies into standard clinical protocols, this phase necessitates precise planning and thorough review. The implementation of neuroprotection strategies calls for the creation of standardized guidelines and protocols that complement current therapeutic modalities. Validating the safety, effectiveness, and practicability of these therapies calls for extensive clinical trials and real-world investigations to bridge the gap between laboratory research and clinical realities. Neurologists, neurosurgeons, rehabilitation experts, and other medical professionals must work well together and communicate well to develop a coordinated strategy that maximizes patient care while maximizing the potential advantages of neuroprotection techniques.
Challenges in implementation and personalized approaches
Despite the great potential of neuroprotection techniques, their effective application faces several difficulties. Variability in the patient’s characteristics, the severity of the injury, and the patient’s reaction to therapy emphasize the need for individualized strategies suited to each TBI case. Getting over logistical problems, gathering funds, and educating healthcare professionals are necessary for integrating neuroprotection techniques into a wide range of clinical settings. Adoption of novel interventions necessitates a difficult balancing act between the need for quick deployment of efficient therapies and the comprehensive assessment necessary for their safe and successful application. The creation of flexible and scalable procedures that take into account the intricate interaction of clinical, logistical, and patient-related elements is essential for overcoming these obstacles.
ICP and CPP offer insights into CBF dynamics. Yet, metabolic events can be assessed through probes, like PbtO2, reflecting extracellular oxygen tension.[
Although the prospect of using enhanced multimodal monitoring to direct care in older patients is enticing, there is little concrete knowledge in this area. This lack of insight can be partially explained by the higher risks associated with invasive intracranial monitoring in older patients, who frequently present taking anticoagulant and antiplatelet medications, in part by the likelihood of a suboptimal outcome, which has led to a lowered frequency of aggressive monitoring and therapy in this population.
DC was performed for the management of unilateral or bilateral brain swelling in TBI patients older than 66 years; unfortunately, in this study, mortality was 77%, and overall unfavorable outcomes occurred in 82%, so this strategy has been discontinued in clinical practice for patients over the age of 65 who present with a GCS of 8 or less.[
Ethical considerations and patient perspectives
In the context of surgical approaches, ethical considerations and the viewpoints of patients are critical factors that significantly influence clinical decisions and treatment strategies. These elements underscore the importance of respecting the autonomy and preferences of patients while ensuring that medical interventions are guided by ethical principles such as beneficence and justice. Furthermore, dealing with intricate ethical dilemmas related to issues such as the appropriateness of medical interventions, allocation of resources, and the influence of cultural beliefs necessitates a careful and patient-centric approach. By involving patients and their families in shared decision-making, offering comprehensive information, and advocating for their needs, healthcare providers can effectively navigate these ethical complexities and enhance patient outcomes.
CONCLUSION
TBI remains a significant public health concern, and researchers have tirelessly explored diverse therapeutic approaches to mitigate its debilitating consequences. The diverse range of neuroprotective strategies underscores the complexity of TBI management. Even if neurosurgery plays a pivotal role in TBI management, no single intervention appears to provide a panacea for this multifaceted condition. Instead, a holistic approach that considers patient-specific factors, timing, and a combination of therapies may hold the key to improving outcomes. Furthermore, ongoing efforts to standardize protocols and refine patient selection criteria will contribute to more reliable outcomes in future treatments.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al Mamun A, Ngwa C, Qi S, Honarpisheh P, Datar S, Sharmeen R. Neuronal CD200 signaling is protective in the acute phase of ischemic stroke. Stroke. 2021. 52: 3362-73
2. Bohman LE, Schuster JM. Decompressive craniectomy for management of traumatic brain injury: An update. Curr Neurol Neurosci Rep. 2013. 13: 392
3. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of acute epidural hematomas. Neurosurgery. 2006. 58: S7-15
4. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of acute subdural hematomas. Neurosurgery. 2006. 58: S16-24 discussion Si-iv
5. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006. 58: S2-46
6. Cameron JL, Cameron AM, editors. The management of traumatic brain injury. Current surgical therapy. Netherlands: Elsevier; 2017. p.
7. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017. 80: 6-15
8. Carter E, Coles JP. Imaging in the diagnosis and prognosis of traumatic brain injury. Expert Opin Med Diagn. 2012. 6: 541-54
9. Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004. 43: 28-60
10. Chandra VV, Mowliswara Prasad BC, Banavath HN, Chandrasekhar Reddy K. Cisternostomy versus decompressive craniectomy for the management of traumatic brain injury: A randomized controlled trial. World Neurosurg. 2022. 162: e58-64
11. Cherian I, Beltran M, Landi A, Alafaci C, Torregrossa F, Grasso G. Introducing the concept of “CSF-shift edema” in traumatic brain injury. J Neurosci Res. 2018. 96: 744-52
12. Cherian I, Beltran M, editors. A unified physical theory for CSF circulation, cooling and cleaning of the brain, sleep, and head injuries in degenerative cognitive disorders. Berlin: Springer International Publishing; 2017. p. 773-83
13. Cherian I, Bernardo A, Grasso G. Cisternostomy for traumatic brain injury: Pathophysiologic mechanisms and surgical technical notes. World Neurosurg. 2016. 89: 51-7
14. Cherian I, Burhan H. Outcomes of severe head injury patients undergoing cisternostomy at tertiary care hospital in Nepal. Indones J Neurosurg. 2019. 2: 55-9
15. Cherian I, Grasso G, Bernardo A, Munakomi S. Anatomy and physiology of cisternostomy. Chin J Traumatol. 2016. 19: 7-10
16. Cherian I, Munakomi S. Surgical technique for cisternostomy: A review. Int J Stud Res. 2013. 3: 5
17. Cherian I, Yi G, Munakomi S. Cisternostomy: Replacing the age old decompressive hemicraniectomy?. Asian J Neurosurg. 2013. 8: 132-8
18. Cooper DJ, Nichol AD, Bailey M, Bernard S, Cameron PA, Pili-Floury S. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury. JAMA. 2018. 320: 2211
19. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011. 364: 1493-502
20. Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015. 72: 355-62
21. De Bonis P, Pompucci A, Mangiola A, Paternoster G, Festa R, Nucci CG. Decompressive craniectomy for elderly patients with traumatic brain injury: It’s probably not worth the while. J Neurotrauma. 2011. 28: 2043-8
22. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019. 130: 1080-97
23. Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015. 127: 3-13
24. Faul M, Wald MM, Xu L, Coronado VG, editors. Traumatic brain injury in the United States; Emergency department visits, hospitalizations, and deaths, 2002-2006. US: National Center for Injury Prevention and Control; 2010. p.
25. Feigin VL, Theadom A, Barker-Collo S, Starkey NJ, McPherson K, Kahan M. Incidence of traumatic brain injury in New Zealand: A population-based study. Lancet Neurol. 2013. 12: 53-64
26. Fletcher JL, Dill LK, Wood RJ, Wang S, Robertson K, Murray SS. Acute treatment with TrkB agonist LM22A-4 confers neuroprotection and preserves myelin integrity in a mouse model of pediatric traumatic brain injury. Exp Neurol. 2021. 339: 113652
27. Gao T, Chen Z, Chen H, Yuan H, Wang Y, Peng X. Inhibition of HMGB1 mediates neuroprotection of traumatic brain injury by modulating the microglia/macrophage polarization. Biochem Biophys Res Commun. 2018. 497: 430-6
28. Gean AD, Fischbein NJ. Head trauma. Neuroimaging Clin N Am. 2010. 20: 527-56
29. Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012. 4: 134ra60
30. Gupte R, Christian S, Keselman P, Habiger J, Brooks WM, Harris JL. Evaluation of taurine neuroprotection in aged rats with traumatic brain injury. Brain Imaging Behav. 2019. 13: 461-71
31. Hanscom M, Loane DJ, Shea-Donohue T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J Clin Invest. 2021. 131: e143777
32. Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci. 2020. 40: 2960-74
33. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016. 375: 1119-30
34. James SL, Theadom A, Ellenbogen RG, Bannick MS, MountjoyVenning WC, Lucchesi LR. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019. 18: 56-87
35. Jiang JY, Xu W, Li WP, Xu WH, Zhang J, Bao YH. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain Injury: A multicenter, prospective, randomized controlled study. J Neurotrauma. 2005. 22: 623-8
36. Keating CE, Cullen DK. Mechanosensation in traumatic brain injury. Neurobiol Dis. 2021. 148: 105210
37. Kiening KL, Unterberg AW, Bardt TF, Schneider GH, Lanksch WR. Monitoring of cerebral oxygenation in patients with severe head injuries: Brain tissue PO2 versus jugular vein oxygen saturation. J Neurosurg. 1996. 85: 751-7
38. Kim CC, Nakamura MC, Hsieh CL. Brain trauma elicits non-canonical macrophage activation states. J Neuroinflammation. 2016. 13: 117
39. Kolias AG, Kirkpatrick PJ, Hutchinson PJ. Decompressive craniectomy: Past, present and future. Nat Rev Neurol. 2013. 9: 405-15
40. Kolias AG, Viaroli E, Rubiano AM, Adams H, Khan T, Gupta D. The current status of decompressive craniectomy in traumatic brain injury. Curr Trauma Rep. 2018. 4: 326-32
41. Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014. 76: 845-61
42. Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J. Incidence of traumatic brain injury across the full disease spectrum. Epidemiology. 2011. 22: 836-44
43. Levi V, Vetrano IG. May cisternostomy and glymphatic system be considered the deus ex machina of refractory posttraumatic intracranial hypertension?. World Neurosurg. 2018. 117: 471-2
44. Lipinski DM, Barnard AR, Singh MS, Martin C, Lee EJ, Davies WI. CNTF gene therapy confers lifelong neuroprotection in a mouse model of human retinitis pigmentosa. Mol Ther. 2015. 23: 1308-19
45. Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010. 31: 596-604
46. Lotocki G, de Rivero Vaccari JP, Perez ER, Sanchez-Molano J, Furones-Alonso O, Bramlett HM. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: Effects of post-traumatic hypothermia. J Neurotrauma. 2009. 26: 1123-34
47. Maas AI, Fleckenstein W, de Jong DA, van Santbrink H. Monitoring cerebral oxygenation: Experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir Suppl (Wien). 1993. 59: 50-7
48. McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009. 68: 709-35
49. McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013. 136: 43-64
50. Meixensberger J, Jaeger M, Väth A, Dings J, Kunze E, Roosen K. Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2003. 74: 760-4
51. Mendelow AD, Gregson BA, Rowan EN, Francis R, McColl E, McNamee P. Early surgery versus initial conservative treatment in patients with traumatic intracerebral hemorrhage (STITCH[Trauma]): The first randomized trial. J Neurotrauma. 2015. 32: 1312-23
52. Menon DK, Coles JP, Gupta AK, Fryer TD, Smielewski P, Chatfield DA. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004. 32: 1384-90
53. Meyfroidt G, Taccone FS. Another failed attempt of neuroprotection: Progesterone for moderate and severe traumatic brain injury. Minerva Anestesiol. 2016. 82: 486-91
54. Nangunoori R, Maloney-Wilensky E, Stiefel M, Park S, Andrew Kofke W, Levine JM. Brain tissue oxygen-based therapy and outcome after severe traumatic brain injury: A systematic literature review. Neurocrit Care. 2012. 17: 131-8
55. Ng SY, Lee AY. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019. 13: 528
56. Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD. The international incidence of traumatic brain injury: A systematic review and meta-analysis. Can J Neurol Sci. 2016. 43: 774-85
57. Nozari A, Sharma A, Wang Z, Feng L, Muresanu DF, Tian ZR. Co-administration of nanowired oxiracetam and neprilysin with monoclonal antibodies to amyloid beta peptide and p-tau thwarted exacerbation of brain pathology in concussive head injury at hot environment. Adv Neurobiol. 2023. 32: 271-313
58. Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013. 14: 265-77
59. Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015. 157: 1683-96
60. Pennings FA, Schuurman PR, van den Munckhof P, Bouma GJ. Brain tissue oxygen pressure monitoring in awake patients during functional neurosurgery: The assessment of normal values. J Neurotrauma. 2008. 25: 1173-7
61. Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002. 17: 1137-52
62. Rosenthal G, Hemphill JC, Sorani M, Martin C, Morabito D, Obrist WD. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008. 36: 1917-24
63. Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008. 25: 719-38
64. Samandouras G, editors. Traumatic brain injury. The neurosurgeon’s handbook. Oxford: Oxford University Press; 2010. p. 207-38
65. Sarrafzadeh AS, Kiening KL, Bardt TF, Schneider GH, Unterberg AW, Lanksch WR, editors. Cerebral oxygenation in contusioned vs. Nonlesioned brain tissue: Moniting of PtiO2 with licox and paratrend. Intracranial pressure and neuromonitoring in brain injury. Berlin: Springer Vienna; 1998. p. 186-9
66. Singh SP, Jadhav SH, Chaturvedi CP, Nityanand S. Therapeutic efficacy of multipotent adult progenitor cells versus mesenchymal stem cells in experimental autoimmune encephalomyelitis. Regen Med. 2017. 12: 377-96
67. Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: A cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010. 113: 556-63
68. Spiotta AM, Stiefel MF, Gracias VH, Garuffe AM, Kofke WA, Maloney-Wilensky E. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg. 2010. 113: 571-80
69. Stiell IG, Wells GA, Vandemheen K, Clement C, Lesiuk H, Laupacis A. The Canadian CT head rule for patients with minor head injury. Lancet. 2001. 357: 1391-6
70. Szczygielski J, Mautes AE, Müller A, Sippl C, Glameanu C, Schwerdtfeger K. Decompressive craniectomy increases brain lesion volume and exacerbates functional impairment in closed head injury in mice. J Neurotrauma. 2016. 33: 122-31
71. Tanaka Y, Ohno K. Chronic subdural hematoma-an up-to-date concept. J Med Dent Sci. 2013. 60: 55-61
72. Te Ao B, Brown P, Tobias M, Ameratunga S, Barker-Collo S, Theadom A. Cost of traumatic brain injury in New Zealand: Evidence from a population-based study. Neurology. 2014. 83: 1645-52
73. Tomura S, Nawashiro H, Otani N, Uozumi Y, Toyooka T, Ohsumi A. Effect of decompressive craniectomy on aquaporin-4 expression after lateral fluid percussion injury in rats. J Neurotrauma. 2011. 28: 237-43
74. Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998. 26: 1576-81
75. Van Santbrink H, Maas AI, Avezaat CJ. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996. 38: 21-31
76. Walcott BP, Nahed BV, Sheth SA, Yanamadala V, Caracci JR, Asaad WF. Bilateral hemicraniectomy in non-penetrating traumatic brain injury. J Neurotrauma. 2012. 29: 1879-85
77. Wang M, Luo L. An effective NADPH oxidase 2 inhibitor provides neuroprotection and improves functional outcomes in animal model of traumatic brain injury. Neurochem Res. 2020. 45: 1097-106
78. Wang Y, Guo Q, Wang W, Wang Y, Fang K, Wan Q. Potential use of bioactive nanofibrous dural substitutes with controlled release of IGF-1 for neuroprotection after traumatic brain injury. Nanoscale. 2022. 14: 18217-30
79. Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: Morbidity, mortality, and operative timing. J Neurosurg. 1991. 74: 212-8
80. Wilson L, Stewart W, Dams-O’Connor K, DiazArrastia R, Horton L, Menon DK. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017. 16: 813-25
81. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013. 14: 128-42
82. Zweckberger K, Erös C, Zimmermann R, Kim SW, Engel D, Plesnila N. Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J Neurotrauma. 2006. 23: 1083-93