- Department of Neurosurgery, Centre Clinical de Soyaux, Soyaux, France
- Department of Orthopedics, Hôpital Robert Debré, Paris, France
- Department of Emergency, Urban Hospital Center of Nevers, Nevers, France
- Department of Neurosurgery, Sana Klinikum, Lichtenberg, Berlin, Germany
Correspondence Address:
Keyvan Mostofi, Department of Neurosurgery, Centre Clinical de Soyaux, Soyuax, France.
DOI:10.25259/SNI_740_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Keyvan Mostofi1, Kamran Shirbache2, Ali Shirbacheh3, Morad Peyravi4. Neurosurgical treatment of cerebellar infarct: Open craniectomy versus endoscopic surgery. 29-Nov-2024;15:442
How to cite this URL: Keyvan Mostofi1, Kamran Shirbache2, Ali Shirbacheh3, Morad Peyravi4. Neurosurgical treatment of cerebellar infarct: Open craniectomy versus endoscopic surgery. 29-Nov-2024;15:442. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13256
Abstract
Background: Cerebellar infarction can lead to severe morbidity and mortality. Current surgical options include decompressive craniectomy (DC) and endoscopic minimally invasive evacuation of necrotic tissue (MEN), but no randomized studies compare their outcomes.This study compares outcomes between DC and MEN in patients with cerebellar infarct using the Glasgow Coma Scale (GCS) and Scale for the Assessment and Rating of Ataxia (SARA) scores.
Methods: Retrospective review of 37 patients treated for cerebellar infarct between 2010 and 2020. Patients were divided into DC and MEN groups, with outcome measures assessed postoperatively.
Results: Both techniques produced similar improvements in GCS and SARA scores, though MEN showed faster healing time and shorter surgery duration.
Conclusion: MEN may offer advantages over traditional surgery in terms of healing and shorter operative time, warranting further investigation.
Keywords: Cerebellar infarct, Endoscopic surgery, External ventricular derivation, Minimally invasive surgery, Neurovascular, Suboccipital craniotomy
INTRODUCTION
Cerebellar infarct management remains controversial with no consensus on the best surgical approach. Unlike swollen supratentorial hemispheric ischemic stroke for which several studies confirmed the usefulness of decompressive craniectomy (DC)[
MATERIALS AND METHODS
This retrospective cohort study analyzed patients with cerebellar infarction who underwent surgical intervention at our clinical center from 2010 to 2020. All patients presenting with symptoms such as headache associated with nausea and vomiting, dysarthria, sensory disturbances, and vertigo initially underwent a brain CT scan. Patients were referred by either a neurologist or radiologist, and the confirmation of cerebellar infarction and its precise location was verified with MRI in diffusion mode [
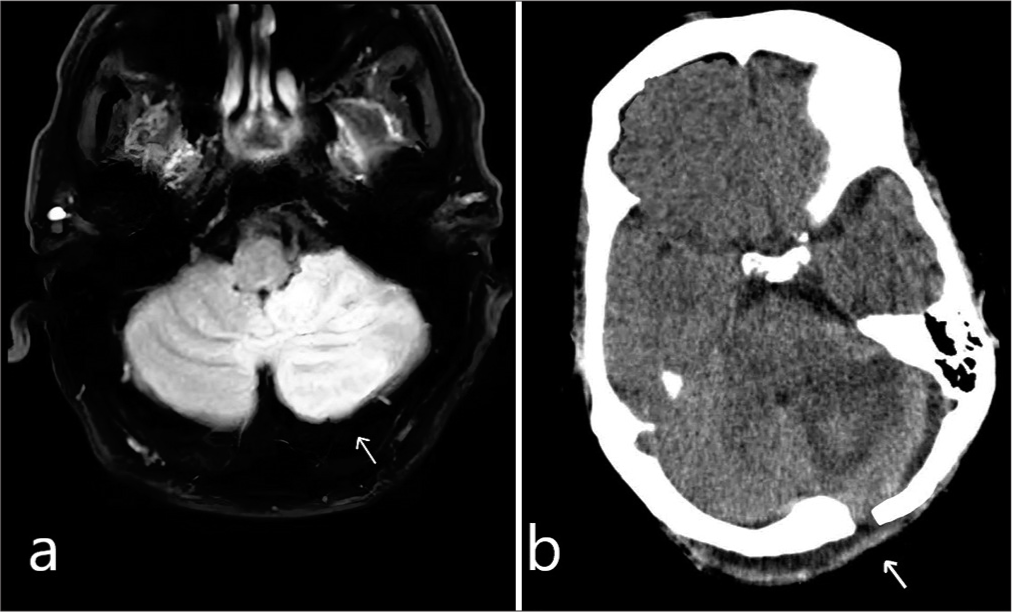
Figure 1:
(a) Axial T1-weighted magnetic resonance imaging scan, the white arrow shows a cerebellar infarction with a measured volume of 7.8 cm3, as determined by the software (preoperative image). (b) Postoperative computed tomography scan demonstrating the removal of necrotic tissue. The white arrow shows the creation of an endoscopic fenestration.
All the patients were treated at a single institution with available follow-up data. The study included 37 patients who underwent surgery for cerebellar infarct, with exclusions for brainstem involvement or secondary lesions. The intervention groups were 20 patients for DC and 17 for MEN.
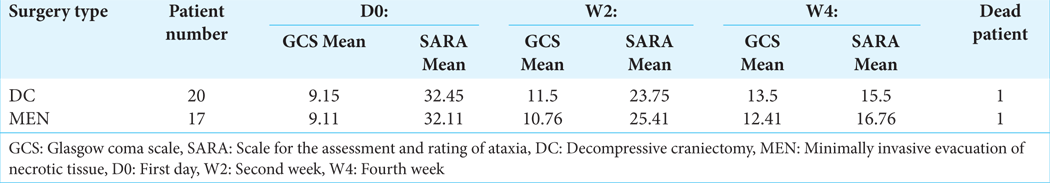
The primary outcomes evaluated in this study were GCS and Scale for the Assessment and Rating of Ataxia (SARA) scores assessed preoperatively, at 2 weeks, and 4 weeks postoperatively. Data were extracted from patient medical records, including clinical presentation, surgical approach, and follow-up assessments [
Technical note: MEN
Since 2014, we have used, more often than not, a MEN. The patient is in the prone position. According to localization and laterality of necrotic lesion, a 2-cm long paramedian vertical-straight incision 2 or 3 cm from the midline is performed [
Figure 3:
(a) Minimally invasive evacuation of necrotic tissue surgery description. (b) Open craniectomy method with 10 cm incision. (c) View of surgery field in an endoscopic method: The yellow arrow indicates dura, the green arrow indicates necrotic tissue and the blue line indicates the keyhole diameter.
RESULTS
DISCUSSION
MEN is a surgical alternative to classical craniectomy for swollen cerebellar and posterior fossa infarct. We have used this method since 2014. We believe that with this technique, the magnitude of the surgery is less. It seeks to avoid major surgery in this category of patients who are generally relatively aged and in a poor general condition. We performed a linear incision from inion to spinous process of C3 or C4 (8–9 cm) in patients operated by DC. For MEN patients, we carried out a 3 cm linear left or right paramedian incision, depending on the side of the lesion. In all patients, the wound was closed by simple interrupted sutures. In patients operated by DC, 10 patients (50%) presented delayed healing of the wound. In general, the wounds were oozing with serosity, requiring different treatments. Therefore, wound care had to be continued for 18–32 days. In MEN-operated patients, we have only two patients who presented delayed wound healing, and wound care had to be continued in some cases for 15–19 days. The average duration of healing in the DC patients was 21 days. The mean duration of healing in the MEN patients was 13 days. We used the two tools of assessment for this study. GCS for the level of consciousness and SARA scale to quantify the functional disabilities experienced by these patients with Posterior Fossa Syndrome (PFS). The analysis and comparison of the results of operated patients in each group before and after surgery show a substantially similar outcome. DC patients gained an average of 2 points 2 weeks and 4 points 4 weeks after surgery in GCS. In MEN patients, the increase in score points was 2.50 in 2 weeks and 3.3 in 4 weeks after surgery. As for the scale of SARA, DC-operated patients had a decreased score of 8.7 (21.75%) 2 weeks and 16.95 (42.38%) 4 weeks after surgery. MEN patients had a decreased score of 6.7 (16.75%) 2 weeks and 15.35 (38.38%) 4 weeks after surgery. The average length of surgery was 91 min in DC patients versus 69 min in MEN patients. In endoscopic technique, the surgery is much faster than traditional surgery. The only additional act in endoscopic surgery is preoperative fluoroscopy to ensure that the lesion is targeted. This act lasts an average of 3 min [
Our findings indicate that both surgical techniques led to comparable outcomes in terms of neurological status and functional disability. Specifically, patients in both groups showed improvement in GCS scores over time, with MEN patients demonstrating slightly higher increases compared to DC patients. Similarly, both groups experienced reductions in SARA scores postoperatively, indicating improved functional disability, with slightly greater improvements observed in DC patients.
To consider the limitations, the sample size of each group was relatively small in our project. Furthermore, the study focused on short-term outcomes immediately postsurgery and did not assess long-term neurological recovery or functional outcomes.
While our findings suggest that MEN may offer advantages over traditional DC in terms of surgical magnitude and wound healing, further research with larger sample sizes and longer follow-up periods is needed to confirm these findings. In addition, future studies should consider the inclusion of a control group and multivariate analysis to account for potential confounders and biases. Nevertheless, our study contributes valuable insights into the comparative effectiveness of MEN versus DC for cerebellar infarct patients, highlighting the need for further investigation in this area.
The small sample size nature of the study may limit the generalizability of the study results. In addition, the study population consisted of patients with cerebellar infarct who underwent surgery at a single institution, which may not be representative of all patients with similar conditions. Therefore, caution should be exercised when extrapolating the findings to other populations or clinical settings.
CONCLUSION
We believe that the evacuation of infarcted tissue by endoscopy provides an interesting alternative to conventional surgery. The clinical outcome is almost identical; however, in terms of healing and the length of the surgery, the outcome is more interesting. MEN presents a promising alternative to traditional DC for cerebellar infarct, with comparable clinical outcomes and benefits such as faster recovery and shorter surgical time.
Ethical approval
Institutional Review Board approval is not required as it is retrospective study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Auer L, Auer T, Sayama I. Indications for surgical treatment of cerebellar haemorrhage and infarction. Acta Neurochir. 1986. 79: 74-9
2. Frank JI, Schumm LP, Wroblewski K, Chyatte D, Rosengart AJ, Kordeck C. Hemicraniectomy and durotomy upon deterioration from infarction-related swelling trial: Randomized pilot clinical trial. Stroke. 2014. 45: 781-7
3. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): A multicentre, open, randomised trial. Lancet Neurol. 2009. 8: 326-33
4. Ioannides K, Tadi P, Naqvi IA, editors. Cerebellar infarct. Treasure Island, FL: StatPearls Publishing; 2017. p.
5. Ito M, Sonokawa T, Mishina H, Hishii M, Sato K. Surgical management of comatose patients with cerebellar infarction. J Clin Neurosci. 1994. 1: 251-6
6. Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY) a randomized, controlled trial. Stroke. 2007. 38: 2518-25
7. Jüttler E, Schweickert S, Ringleb PA, Huttner HB, Köhrmann M, Aschoff A. Long-term outcome after surgical treatment for space-occupying cerebellar infarction: Experience in 56 patients. Stroke. 2009. 40: 3060-6
8. Jüttler E, Unterberg A, Woitzik J, Bösel J, Amiri H, Sakowitz OW. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014. 370: 1091-100
9. Macdonell RA, Kalnins RM, Donnan GA. Cerebellar infarction: Natural history, prognosis, and pathology. Stroke. 1987. 18: 849-55
10. Mostofi K. Neurosurgical management of massive cerebellar infarct outcome in 53 patients. Surg Neurol Int. 2013. 4: 28
11. Reinink H, Jüttler E, Hacke W, Hofmeijer J, Vicaut E, Vahedi K. Surgical decompression for space-occupying hemispheric infarction: A systematic review and individual patient meta-analysis of randomized clinical trials. JAMA Neurol. 2021. 78: 208-16
12. Tohgi H, Takahashi S, Chiba K, Hirata Y. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. The Tohoku cerebellar infarction study Group. Stroke. 1993. 24: 1697-701
13. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 2007. 38: 2506-17
14. Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT. Recommendations for the management of cerebral and cerebellar infarction with swelling: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014. 45: 1222-38