- Department of Neurosurgery, University of Baghdad, College of Medicine, Baghdad, Iraq
- Department of Neurosurgery, University of Mustansiriyah, College of Medicine, Baghdad, Iraq,
- Department of Neurosurgery, Saudi German hospital Ajman, United Arab Emirates,
- Department of Neurosurgery, Hannover Medical School, Hannover, Germany,
- Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq,
- Department of Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, PA, United States.
Correspondence Address:
Samer S. Hoz, Department of Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, PA, United States.
DOI:10.25259/SNI_598_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Muthana1, Mustafa R. Al-Gertani2, Fatimah Oday Ahmed2, Almutasimbellah K. Etaiwi3, Sajjad G. Al-Badri1, Oday Atallah4, Mostafa H. Algabri1, Mustafa Ismail5, Samer S. Hoz6. Occipital artery: Anatomical variations and neurosurgical applications. 01-Sep-2023;14:313
How to cite this URL: Ahmed Muthana1, Mustafa R. Al-Gertani2, Fatimah Oday Ahmed2, Almutasimbellah K. Etaiwi3, Sajjad G. Al-Badri1, Oday Atallah4, Mostafa H. Algabri1, Mustafa Ismail5, Samer S. Hoz6. Occipital artery: Anatomical variations and neurosurgical applications. 01-Sep-2023;14:313. Available from: https://surgicalneurologyint.com/surgicalint-articles/12518/
Abstract
Background: The occipital artery (OA) is a branch of the external carotid artery. It gives rise to several cutaneous, muscular, and meningeal branches to supply different anatomical areas. The implication of OA in the neurosurgical field is well-established in the literature. Our aim in this study is to draw a complete picture of the anatomical variations and neurosurgical applications of the OA.
Methods: A literature review was conducted in Google Scholar and PubMed to review the studies discussing OA, its anatomical variation, and neurosurgical applications.
Results: We identified 29 articles that discuss the anatomical variations and neurosurgical applications of the OA. Certain variables are used to describe the surgical anatomy of OA. We also discussed certain applications of OA and its importance in neurosurgical bypass, embolization, and aneurysms.
Conclusion: Comprehending the anatomy of the OA is crucial for neurosurgeons to safely and effectively perform procedures such as bypass and embolization. In addition, knowledge of the anatomical variations of the OA can help surgeons anticipate potential challenges and tailor their approach accordingly.
Keywords: Anatomical variations, Bypass, Neuroanatomy, Occipital artery
INTRODUCTION
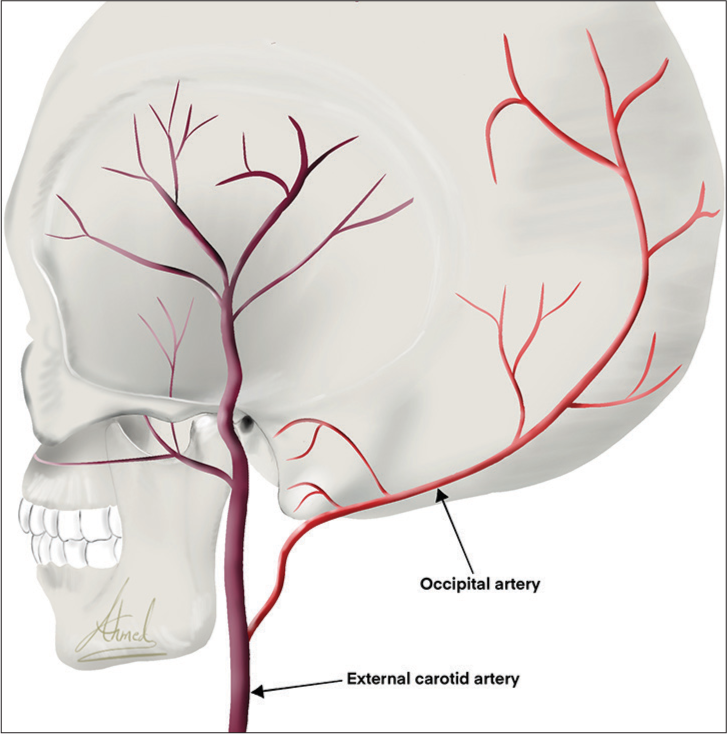
The occipital artery (OA) originates from the posterior part of the external carotid artery (ECA).[
The neurosurgical importance of OA arises from its role in the bypass. The OA gives bypass to the posterior inferior cerebellar artery (PICA), anterior inferior cerebellar artery (AICA), posterior cerebral artery (PCA), and middle cerebral artery (MCA).[
The implication of OA in the neurosurgical field is well-established in the literature. However, we were unable to find studies that provide a collective and cumulative description of the anatomy and clinical applications of OA. Our aim in this study is to draw a complete picture of the anatomical variations and neurosurgical applications of the OA.
MATERIALS AND METHODS
A literature review was conducted in Google Scholar and PubMed to review the studies discussing OA, its anatomical variation, and neurosurgical applications. The following search terms were used: “occipital artery vascular anatomy” and “occipital artery neurosurgical applications.” Inclusion criteria were as follows: (i) English language and (ii) suitable methodology of the targeted data. We exclude studies with the following criteria (i) non-English papers and (ii) questionable results. Results were categorized and selected appropriately. The data extraction includes surgical anatomy and neurosurgical applications of the OA.
RESULTS
On reading and reviewing the available articles and original works regarding OA, concerning the inclusion and exclusion criteria, we identified 29 articles that discuss the anatomical variations and neurosurgical applications of the OA. Certain variables are used to describe the surgical anatomy of OA including origin, course, branches, diameter, and length. We also discussed certain applications of OA and its importance in neurosurgical bypass, embolization, and aneurysms.
DISCUSSION
OA anatomy
OA origin
The OA is a branch of the ECA. It normally originates from the posterior aspect of the ECA opposite to the facial artery. Recent evidence also reflects that OA may infrequently originate from the internal carotid artery (ICA) while OA that originates from the vertebral artery is a highly rare phenomenon.[
OA course
The initial segment of OA is straight as it travels up through the upper neck, then the vessel becomes more torturous and provides a more redundant blood supply as it travels up the posterior scalp.[
The OA has three segments based on the vertical muscle layers through which they run; subcutaneous, transitional, and intramuscular. OA courses 81.9 mm from the digastric groove as it exists to the superior nuchal line, then it travels superiorly and posteriorly, passing superficially to the ICA and the vagus nerve, before anastomosing with the deep cervical artery as it courses just beneath the mastoid process of the temporal bone. It, then, travels deep to the sternocleidomastoid and splenius capitis muscles until it reaches the occipital bone’s superior nuchal line, where it then travels superficially to the semispinalis capitis muscle. After it has coursed about halfway across the semispinalis capitis and just lateral of the trapezius, the artery then courses vertically up the back of the head superficial to the occipital belly of the occipitofrontalis. It ascends tortuously up the head in the superficial fascia, anastomosing with a branch of the posterior auricular artery. Then, at the crown of the head, it anastomoses with the parietal branch of the superficial temporal artery.[
OA branches
The OA gives cutaneous branches that supply the occipital area. In addition, it gives rise to several other branches, including the auricular, mastoid, descending, meningeal, muscular, and sternocleidomastoid branches [
The descending branch is the largest division and contains a deep and superficial portion. The superficial portion provides blood to the trapezius, and the deep portion anastomoses with a branch of the costocervical trunk, providing collateral circulation through the ECA and the subclavian artery.
The meningeal branch supplies the posterior cranial fossa dura mater. The muscular branches supply several muscles along the course of the OA, including the digastric and longus capitis muscles. The sternocleidomastoid branch divides the carotid triangle into upper and lower branches and supplies the muscle that is named after it.[
OA diameter and length
The mean diameter of OA as it exits from the digastric groove is about 2.05 mm, and at the level of the superior nuchal line, is approximately 2.01 mm.[
Neurosurgical application of OA
Bypass
The OA is an important donor artery for posterior fossa revascularization due to its size, anatomical proximity to target recipient vessels, and flow rate. In addition, the OA has been reported to provide a mean blood flow of 15–80 mL/min when used for posterior fossa bypass.[
OA-PICA bypasses
It is the first intracranial posterior circulation revascularization procedure to treat vertebrobasilar insufficiency, as reported by Ausman et al.[
Kawashima et al. conducted an anatomical study of OA-PICA bypasses, which showed several advantages: (1) The recipient vessel, which is usually the caudal loop of the PICA, has a rather large diameter, thus making the anastomosis easier. (2) The lumen caliber of the donor vessel, which is usually the OA, closely approximates that of the recipient vessel. (3) The bypass surgery can be performed in a shallow and wide operative field, which makes the procedure easier.[
The caudal loop of the PICA which is a portion of the tonsillomedullary segment is generally considered to be the optimal site for OA-PICA anastomosis, and its inferomedial location facilitates anastomosis with adequate intraoperative maneuverability and only minimal retraction of the cerebellar tonsil.[
Lister et al. described two anatomical variants of PICA with absent caudal loops, both of which take on a straight course.[
OA-PCA bypass
OA-PCA bypass is used as an alternative to conventional superficial temporal artery-superior cerebellar artery bypass or in the event that PCA bypasses are unavailable. A few cases of OA-PCA interposition graft bypass through the occipital interhemispheric approach have been reported for vertebral artery occlusion and PCA aneurysms.[
OA-MCA bypass
OA-MCA bypass is a less commonly used procedure; however, it can be effective when the posterior region of the brain requires an additional source of blood supply, and this bypass is preferred in the lateral position, with the head slightly rotated toward the contralateral side to expose the surgical region, which helps to decrease the risk of high intracranial pressure and brain bulging.[
In addition, it could be beneficial to obtain a large craniotomy and to combine the OA-MCA bypass with indirect revascularization procedures such as encephaloduroarteriosynangiosis or pericranial flap; therefore, OA-MCA bypass has been implicated in the treatment of moyamoya disease.[
OA-AICA bypass
This anastomosis is rarely performed because the AICA is too thin, causing it to be mismatched with the OA.[
OA embolization
Therapeutic embolization of the ECA or its branches is widely accepted as a definitive treatment or an adjunct surgical procedure in patients with highly vascular craniofacial lesions.[
This procedure is generally considered to be safe and without risks, although complications such as facial palsy, intracranial stroke, and tissue necrosis may occur.[
OA aneurysm
The true aneurysms are caused by a defect in the wall of the vessels. In contrast, pseudoaneurysms, which are also known as false aneurysms, are the result of a vessel wall rupture, often secondary to trauma.[
CONCLUSION
Being acquainted with the variable anatomy of the OA is crucial for successful neurosurgical bypass, embolization, and various treatment modalities of aneurysms. The need for more cadaver research may help neurosurgeons gain a more comprehensive understanding of the OA‘s anatomy and its variations. This can provide valuable insights into the optimal placement and trajectory of bypass grafts, minimizing the risk of postoperative complications.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alvernia JE, Fraser K, Lanzino G. The occipital artery: A microanatomical study. Neurosurgery. 2006. 58: S114-22
2. Ateş O, Ahmed AS, Niemann D, Baskaya MK. The occipital artery for posterior circulation bypass: Microsurgical anatomy. Neurosurg Focus. 2008. 24: E9
3. Ausman JI, Diaz FG, Vacca DF, Sadasivan B. Superficial temporal and occipital artery bypass pedicles to superior, anterior inferior, and posterior inferior cerebellar arteries for vertebrobasilar insufficiency. J Neurosurg. 1990. 72: 554-8
4. Ausman JI, Lee MC, Klassen AC, Seljeskog EL, Chou SN. Stroke: What‘s new?. Cerebral revascularization. Minn Med. 1976. 59: 223-7
5. Bitoh S, Hasegawa H, Obashi J, Maruno M. Sudden appearance of occipital-vertebral arterial anastomoses during therapeutic embolization. Surg Neurol. 1985. 24: 160-4
6. Cetin N, Akkan K, Ucar M, Onal B, Ilgit E. Bilateral occipital arteries of internal carotid origin: Report of a case and review of the literature. J Belg Soc Radiol. 2015. 99: 69-71
7. Crowley RW, Medel R, Dumont AS. Operative nuances of an occipital artery to posterior inferior cerebellar artery bypass. Neurosurg Focus. 2009. 26: E19
8. Fujimura M, Inoue T, Shimizu H, Tominaga T. Occipital artery-anterior inferior cerebellar artery bypass with microsurgical trapping for exclusively intra-meatal anterior inferior cerebellar artery aneurysm manifesting as subarachnoid haemorrhage. Case report. Neurol Med Chir (Tokyo). 2012. 52: 435-8
9. Fukuda H, Evins AI, Burrell JC, Stieg PE, Bernardo A. A safe and effective technique for harvesting the occipital artery for posterior fossa bypass surgery: A cadaveric study. World Neurosurg. 2014. 82: e459-65
10. Graefe S, Tadi P, editors. Neuroanatomy. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. p.
11. Iwai T, Izumi T, Inoue T, Maegawa J, Mitsudo K, Tohnai I. Incidence of the occipital artery arising from the internal carotid artery identified by three-dimensional computed tomographic angiography. Br J Oral Maxillofac Surg. 2012. 50: 373-5
12. John N, Leach JL, Rachana T, Mangano FT. Traumatic aneurysm of the occipital artery secondary to paintball injury. Clin Neurol Neurosurg. 2009. 111: 105-8
13. Kawashima M, Rhoton AL, Tanriover N, Ulm AJ, Yasuda A, Fujii K. Microsurgical anatomy of cerebral revascularization. Part II: Posterior circulation. J Neurosurg. 2005. 102: 132-47
14. Kazumata K, Kamiyama H, Saito H, Maruichi K, Ito M, Uchino H. Direct anastomosis using occipital artery for additional revascularization in moyamoya disease after combined superficial temporal artery-middle cerebral artery and indirect bypass. Oper Neurosurg (Hagerstown). 2017. 13: 213-23
15. Kerber CW. Flow-controlled therapeutic embolization: A physiologic and safe technique. AJR Am J Roentgenol. 1980. 134: 557-61
16. Khodadad G, Singh RS, Olinger CP. Possible prevention of brain stem stroke by microvascular anastomosis in the vertebrobasilar system. Stroke. 1977. 8: 316-21
17. Korinth MC, Thron A, Bertalanffy H, Gilsbach JM. Coil embolization of an incidental posterior cerebral artery aneurysm after initial OA-PCA bypass surgery. Zentralbl Neurochir. 2000. 61: 158-61
18. Lister JR, Rhoton AL, Matsushima T, Peace DA. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982. 10: 170-99
19. Mahalley MS, Boone SC. External carotid-cavernous fistula treated by arterial embolization. Case report. J Neurosurg. 1974. 40: 110-4
20. Mohit AA, Eskridge J, Ellenbogen R, Shaffrey CI. Aneurysmal bone cyst of the atlas: Successful treatment through selective arterial embolization: Case report. Neurosurgery. 2004. 55: 982
21. Ostrowski P, Bonczar M, Plutecki D, Kwiecińska M, Rams D, Dziedzic M. The occipital artery: A meta-analysis of its anatomy with clinical correlations. Anat Sci Int. 2023. 98: 12-21
22. Patel M, Tchelepi H, Rice DH. Traumatic pseudoaneurysm of the occipital artery: Case report and review of the literature. Ear Nose Throat J. 2008. 87: E7-12
23. Reis CV, Safavi-Abbasi S, Zabramski JM, Gusmão SN, Spetzler RF, Preul MC. The history of neurosurgical procedures for moyamoya disease. Neurosurg Focus. 2006. 20: E7
24. Sakharpe AK, Caseclla M, editors. SteatPearls. Treasure Island, FL: SteatPearls Publishing; 2021. p.
25. Spetzler RF, Modic M, Bonstelle C. Spontaneous opening of large occipital-vertebral artery anastomosis during embolization. Case report. J Neurosurg. 1980. 53: 849-50
26. Sundt TM, Piepgras DG. Occipital to posterior inferior cerebellar artery bypass surgery. J Neurosurg. 1978. 48: 916-28
27. Touho H, Karasawa J, Ohnishi H, Nakase H, Watabe Y, Yamada K. Anastomosis of occipital artery to anterior inferior cerebellar artery with interposition of superficial temporal artery. Case report. Surg Neurol. 1993. 40: 164-70
28. Touho H, Karasawa J, Ohnishi H, Kobitsu K. Anastomosis of occipital artery to posterior cerebral artery with interposition of superficial temporal artery using occipital interhemispheric transtentorial approach: Case report. Surg Neurol. 1995. 44: 245-9
29. Yang HJ, Choi YH. Posttraumatic pseudoaneurysm in scalp treated by direct puncture embolization using N-butyl-2-cyanoacrylate: A case report. Korean J Radiol. 2005. 6: 37-40