- Department of Neurosurgery, Suwa Red Cross Hospital, Kogandori, Suwa, Nagano, Japan
Correspondence Address:
Yukinari Kakizawa
Department of Neurosurgery, Suwa Red Cross Hospital, Kogandori, Suwa, Nagano, Japan
DOI:10.4103/sni.sni_20_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Masahito Katsuki, Yasunaga Yamamoto, Naomichi Wada, Yukinari Kakizawa. Occipital artery to extracranial vertebral artery anastomosis for bilateral vertebral artery stenosis at the origin: A case report. 16-Apr-2018;9:82
How to cite this URL: Masahito Katsuki, Yasunaga Yamamoto, Naomichi Wada, Yukinari Kakizawa. Occipital artery to extracranial vertebral artery anastomosis for bilateral vertebral artery stenosis at the origin: A case report. 16-Apr-2018;9:82. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8846
Abstract
Background:Revascularization of posterior circulation is essential in patients with severe bilateral vertebral artery (VA) stenosis despite administering maximal medical treatment, due to the high mortality of posterior circulation stroke.
Case Description:We present a 69-year-old man with bilateral severe VA stenosis at the origins, treated with occipital artery (OA)-distal VA anastomosis.
Conclusion:Endovascular treatment and other surgical treatments, such as bypass grafting, are effective, but OA-VA anastomosis is a safe and effective procedure for revascularization of the posterior circulation.
Keywords: Arterial reconstruction, occipital artery-vertebral artery transposition, vertebral artery stenosis, vertebrobasilar insufficiency
INTRODUCTION
Bilateral vertebral artery (VA) stenosis can cause posterior circulatory insufficiency when the posterior communicating artery or other collaterals are poorly developed. The proximal portion, especially the origin, of the VA is the most common location (92%) of atherosclerotic occlusive disease in VA.[
CASE DESCRIPTION
Case Report
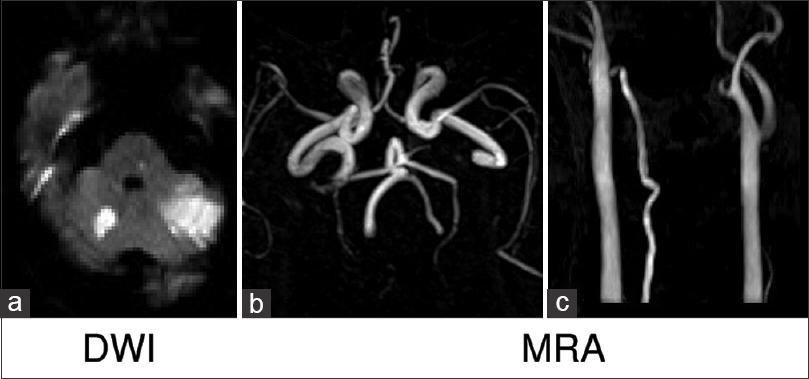
A 69-year-old man was admitted to our hospital because of bilateral ataxia and dizziness. He had not been taking any medications and had been smoking 40 cigarettes per day for 49 years. Laboratory tests revealed mild dyslipidemia and impaired glucose tolerance. Blood pressure on admission was 188/92 mmHg. Magnetic resonance imaging (MRI) showed acute brain infarction at the bilateral cerebellar hemispheres and the right thalamus. The lesions in the cerebellum were not perfectly consistent with vessel-dominant regions supplied by the anterior inferior cerebellar artery or posterior inferior cerebellar artery. Head magnetic resonance angiography (MRA) showed bilateral VAs but posterior communicating arteries were not observed bilaterally. Neck MRA indicated right VA but did not show left VA [
Figure 1
Diffusion weighted magnetic resonance imaging showed acute brain infarction at the bilateral cerebellar hemispheres and the right thalamus (a). Head MRA showed the bilateral VAs but posterior communicating arteries were not observed bilaterally (b). Neck MRA indicated the right VA but not the left VA (c)
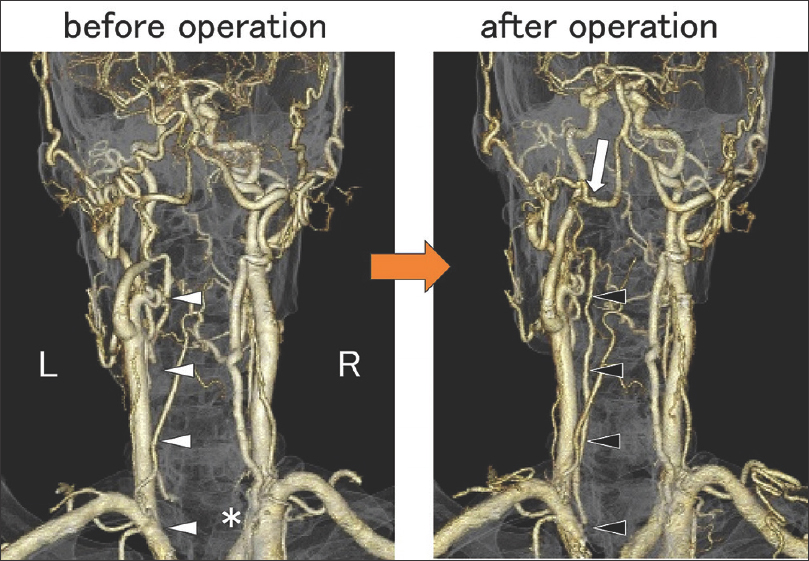
Figure 2
Posterior view of contrast-enhanced 3D-CTA before the operation (left) showed right VA severe stenosis at the origin (asterisk) and did not show the left VA from the ostia to the lower edge of the second cervical vertebra (white arrowheads). Whereas, 3D-CTA just after the operation (right) showed that the anastomosis was patent (white arrow) and the left VA was shown from the ostia to the union (black arrowheads)
We planned surgical reconstruction of posterior circulation in order to avoid fatal brain infarction due to posterior circulatory insufficiency, which is related to high morbidity and mortality. In terms of preoperative neurological symptoms, the muscle tones of extremities were intact. The gait on a flat surface was normal without assistance, but the tandem gait was unsteady when attempting to touch the heel of the right foot to the toe of the left foot. Mild dysmetria was seen when finger–nose–finger test was performed with the left forearm. He felt a difficulty to use his left hand as a subjective symptom. Dysarthria was mild to moderate with some slurring of speech so that we asked the patient to repeat sometimes.
Operation
We performed left OA to left distal extracranial VA anastomosis 3 months after the first admission avoiding hemorrhagic infarction [
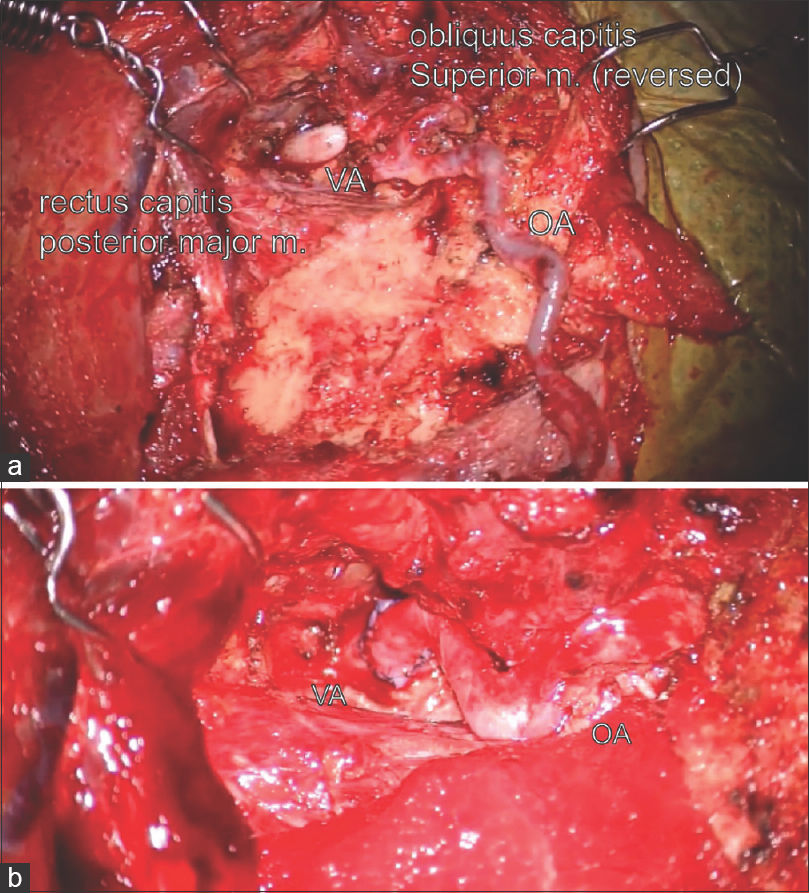
Figure 3
Intraoperative findings of left OA to left extracranial VA anastomosis. After dissecting the OA and the VA (a), the OA was mobilized and anastomosed end-to-side to the VA (b). The VA was observed in the occipito-atlantal (C0-C1) level within the suboccipital triangle bounded by the rectus capitis posterior major muscle, obliquus capitis superior muscle, and obliquus capitis inferior muscle [
Postoperative course
On the day following the operation, the patient walked without assistance but watching and the bilateral ataxia improved. 3D-CTA just after the operation showed that the anastomosis was patent, and the left VA was observed from the ostia to the union [
DISCUSSION
Posterior circulatory insufficiency carries risks of severe morbidity and mortality. Up to 25% of transient ischemic attacks (TIAs) and strokes are associated with posterior circulation, and patients with symptomatic VA stenosis have a 30–35% risk of stroke over 5 years and a 5–11% combined risk of stroke and death at 1 year.[
Open surgery and/or endovascular treatment are planned to revascularize VA. In terms of endovascular treatment, previous randomized controlled trials (RCTs), such as The Vertebral Artery Stenting Trial (VAST)[
In addition, the rates of perioperative stroke and death are comparable for open versus endovascular approaches. The main difference between surgical and endovascular treatment is durability. The perioperative stroke rates of open surgery and endovascular treatment are almost the same (0–4%). However, the patency rate and stroke-free rate of open surgery are both 90% at 10 years.[
Furthermore, in particular cases with severe VA stenosis, it has been reported that hybrid operations combining surgical manipulation of the proximal VA and endovascular techniques can be safe and effective.[
On the contrary, open surgery largely consists of two procedures: bypass grafting and transposition. Bypass grafting involves carotid bypass to the VA from the ostia to the transverse foramen of C6 (V1) or V3, or subclavian bypass to the V1, with vein grafting. Transposition involves anastomosing VA to ICA, the external carotid artery (ECA), or OA to VA, or VA to the subclavian artery.[
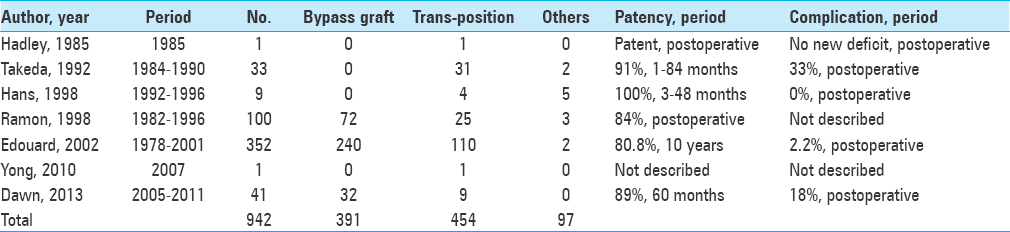
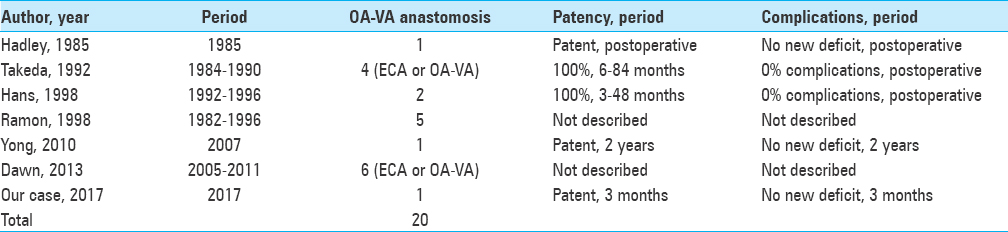
To our knowledge, there have been only 19 previous case reports of OA-VA transposition, as performed in our case. These previous reports indicated good patency and no complications of OA-VA transposition [
In our case, we chose OA-VA anastomosis instead of bypass grafting, VA-subclavian transposition, or endovascular treatment. Bypass grafting requires temporary occlusion of the ICA or common carotid artery (CCA), which markedly reduces cerebral blood flow, and therefore the operation must be performed quickly. Although blood flow in the ICA or CCA can be maintained using an internal shunt tube or partial vessel occlusion with a curved clip, the possibility of blood flow decrease is not zero. Furthermore, bypass grafting requires invasive and complicated procedures, such as wide skin incision, harvesting of the venous graft, two anastomoses, and multiple temporary clipping and occlusion procedures.
VA-subclavian transposition can improve the VA stenosis and was recommended by Ogawa[
Endovascular treatment also requires transient occlusion, so endovascular angioplasty for the right VA is unsafe for the same reason, and angioplasty itself can cause strokes due to embolization of debris despite using an embolic protection device, such as a filter. Endovascular angioplasty for the left VA is also difficult because insertion of the guidewire cannot be performed easily due to near occlusion of the left VA.
Based on the points discussed earlier, we performed left OA-VA anastomosis. Of course, it required temporary occlusion of the left VA. However, the left VA was almost occluded prior to surgery and the right VA could maintain its flow during temporary left VA occlusion. Therefore, OA-VA anastomosis was a safe procedure in this case [
Figure 4
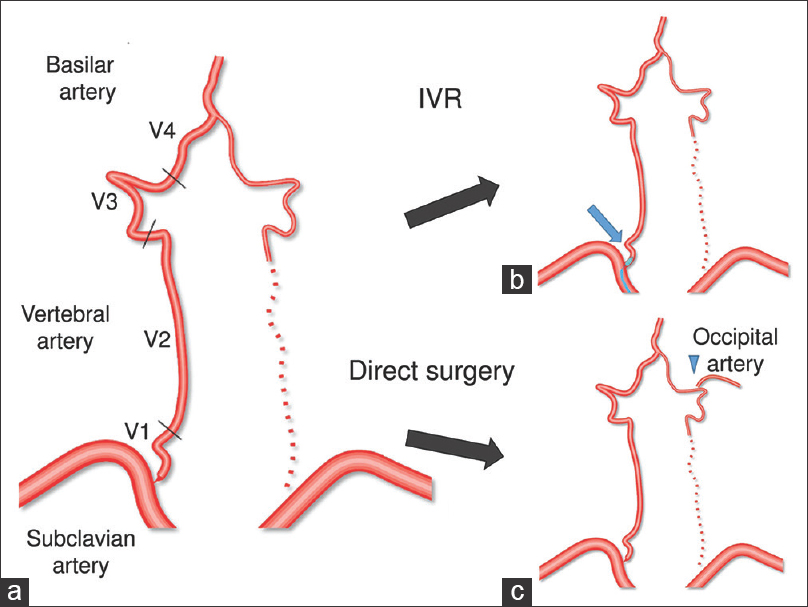
The surgical strategy in this case. Schema of arteries in this case (a). The VA is divided into four segments: V1, origin to transverse foramen of C6; V2, from the transverse foramen of C6 to the transverse foramen of C2; V3, from C2 to the dura; V4, from the dura to their union to form the basilar artery. In our case, the right VA was the solitary feeding artery for the brainstem, so it was difficult to perform procedures requiring temporary occlusion of the right VA, such as anastomosing the right VA to the subclavian artery and endovascular angioplasty for the right VA. In addition, the left VA showed severe stenosis, so anastomosing the left VA to the subclavian artery and endovascular angioplasty for the left VA had no guarantee of success (b). On the contrary, left occipital artery-VA anastomosis was safe. Of course, it required temporary occlusion of the left VA. However, the left VA was almost occluded prior to surgery and the right VA was able to maintain its flow during temporary left VA occlusion (c)
Furthermore, when transposition of V1 to the subclavian artery is needed as a radical operation or two-stage operation, OA-VA anastomosis before V1-subclavian transposition can maintain flow of the VA for the brainstem via anastomosis at V3 during V1-subclavian transposition with temporary occlusion of the proximal VA. Therefore, OA-VA anastomosis can be a safe procedure and lead to radical operation.
CONCLUSION
OA to distal extracranial VA anastomosis is a good candidate for symptomatic bilateral severe VA stenosis at the origin and can be performed more safely and more easily than bypass grafting and endovascular treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abe A, Okubo S, Onozawa S, Nakajima M, Suzuki K, Harada-Abe M. Acute vertebral artery origin occlusion leading to basilar artery thrombosis successfully treated by angioplasty with stenting and thrombectomy. Interv Neuroradiol. 2014. 20: 325-8
2. Antoniou GA, Murray D, Georgiadis GS, Antoniou SA, Schiro A, Serracino-Inglott F. Percutaneous transluminal angioplasty and stenting in patients with proximal vertebral artery stenosis. J Vasc Surg. 2012. 55: 1167-77
3. Berguer R, Flynn LM, Kline RA, Caplan L. Surgical reconstruction of the extracranial vertebral artery: Management and outcome. J Vasc Surg. 2000. 31: 9-18
4. Caplan L. Posterior circulation ischemia: Then, now, and tomorrow. The Thomas Willis Lecture-2000. Stroke. 2000. 31: 2011-23
5. Compter A, van der Worp HB, Schonewille WJ, Vos JA, Boiten J, Nederkoorn PJ. Stenting versus medical treatment in patients with symptomatic vertebral artery stenosis: A randomised open-label phase 2 trial. Lancet Neurol. 2015. 14: 606-14
6. Dawn M, Coleman , Andrea O, Enrique C, Shipra A, Ramon B. Contemporary outcomes after distal vertebral reconstruction. J Vasc Surg. 2013. 58: 152-7
7. Edouard K, Barbara P, Laurent C, Fabien K, Amine B. Distal vertebral artery reconstruction: Long–term outcome. J Vasc Surg. 2002. 36: 549-54
8. Flossmann E, Rothwell PM. Prognosis of vertebrobasilar transient ischemic attack and minor stroke. Brain. 2003. 126: 1940-
9. Hadley MN, Spetzler RF, Masferrer R, Martin NA, Carter LP. Occipital artery to extradural vertebral artery bypass procedure. J Neurosurg. 1985. 63: 622-5
10. Hans JS. Cervical vertebral and subclavian artery reconstruction. Neurol Med Chir. 1998. 38: 289-93
11. Henry M, Polydorou A, Henry I, Ad Polydorou I, Hugel IM, Anagnostopoulou S. Angioplasty and stenting of extracranial vertebral artery stenosis. Int Angiol. 2005. 24: 311-24
12. Heyman A, Wilkinson WE, Hurwitz BJ, Haynes CS, Utley CM, Berguer R, Bauer RB.editors. Vertebrobasilar arterial occlusive disease: Medical and surgical management. Clinical and epidemiologic aspects of vertebrobasilar and nonfocal cerebral ischemia. New York: Raven Press; 1984. p. 27-35
13. Hugh SM, Susanna CL, Wilhelm K, Ursula GS, Ian F, Peter MR. Stenting for symptomatic vertebral artery stenosis: The vertebral Artery Ischaemia Stenting Trial. Neurology. 2017. 89: 1229-36
14. .editorsJapanese guidelines for the Management of Stroke 2015. Tokyo: Kyowa Kikaku; 2015. p. 133-4
15. Jones HR, Millikan CH, Sandok BA. Temporal profile (clinical course) of acute vertebrobasilar system cerebral infarction. Stroke. 1980. 11: 173-
16. Labauge R, Boukobza M, Pagès M, Blard JM, Dimitrijevic J, Salvaing P. Occlusion of the vertebral artery (100 personal cases). Rev Neurol (Paris). 1987. 143: 490-
17. Lee CJ, Morasch M. Treatment of vertebral disease: Appropriate use of open and endovascular techniques. Semin Vasc Surg. 2011. 24: 24-30
18. Mcdowerll FH, Potes J, Groch S. The natural history of internal carotid and vertebral-basilar artery occlusion. Neurology. 1961. 11: 153-7
19. Ogawa A. Surgical reconstruction of the proximal vertebral artery: Vertebral to subclavian transposition. No Shinkei Geka. 1991. 19: 7-13
20. Patrick BK, Ramirez-Lassepas M, Synder BD. Temporal profile of vertebrobasilar territory infarction. Prognostic implications. Stroke. 1980. 11: 643-
21. Radak D, Babic S, Sagic D, Tanaskovic S, Kovacevic V, Otasevic P. Endovascular treatment of symptomatic high-grade vertebral artery stenosis. J Vasc Surg. 2014. 60: 92-7
22. Ramon B, Morasch MD, Kline RA. A review of 100 consecutive reconstructions of the distal vertebral artery for embolic and hemodynamic disease. J Vasc Surg. 1998. 27: 852-9
23. Stayman AN, Nogueira RG, Gupta RA. Systematic review of stenting and angioplasty of symptomatic extracranial vertebral artery stenosis. Stroke. 2011. 42: 2212-
24. Takeda R, Nakagawara J, Tanaka Y, Hashimoto I, Fukuoka S, Sasaki T. Surgical reconstruction of the vertebral artery origin stenosis–Experiences of 33 Cases. Surg Cereb Stroke. 1992. 20: 155-60
25. Whisnant JP, Cartlidge NE, Elveback LR. Carotid and vertebral-basilar transient ischemic attacks: Effect of anticoagulants, hypertension, and cardiac disorders on survival and stroke occurrence- a population study. Ann Neurol. 1978. 3: 107-15
26. Xia L, Yan M, Bin Y, Peng G, Yabing W, Liqun J. Hybrid technique for the treatment of refractory vertebrobasilar insufficiencies. World Neurosurg. 2017. 107: 1051-
27. Yong CK, Chang WO, O-Ki K, Gyojun H. Occipital artery to distal extracranial vertebral artery bypass for bilateral proximal vertebral artery occlusion. Korean J Cerebrovasc Surg. 2010. 12: 57-60