- Department of Neurosurgery, Kagawa Rosai Hospital, Kagawa, Japan
Correspondence Address:
Masatoshi Yunoki
Department of Neurosurgery, Kagawa Rosai Hospital, Kagawa, Japan
DOI:10.4103/2152-7806.188915
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yunoki M, Suzuki K, Uneda A, Yoshino K. Olfactory neuroblastoma followed by emergency surgery for symptomatic intradural spinal metastasis: A case report. Surg Neurol Int 23-Aug-2016;7:77
How to cite this URL: Yunoki M, Suzuki K, Uneda A, Yoshino K. Olfactory neuroblastoma followed by emergency surgery for symptomatic intradural spinal metastasis: A case report. Surg Neurol Int 23-Aug-2016;7:77. Available from: http://surgicalneurologyint.com/surgicalint_articles/olfactory-neuroblastoma-followed-emergency-surgery-symptomatic-intradural-spinal-metastasis-case-report/
Abstract
Background:Olfactory neuroblastoma (ONB) is a rare, aggressive tumor of the nasal cavity. It may invade the paranasal cavities and anterior skull base locally but may also metastasize to the cervical lymph nodes, lungs, or distant central nervous system.
Case Description:Here, we report a case of ONB in which emergency surgery was performed for intradural spinal metastasis (ISM). The patient was a 52-year-old male who underwent surgery for ONB. The tumor extended from the nasal cavity to the intracranial space and was resected completely. After radiotherapy (60 Gy), the patient was discharged without any neurological deficit except anosmia. Seven months after the surgery, he consulted our department because of progressive tetraparesis. Cervical magnetic resonance imaging demonstrated an intradural spinal mass involving C5–T2 and necessitating emergency surgery. The tumor was resected subtotally followed by 58 Gy whole-spine irradiation. The patient's neurological symptoms improved, however, paralysis of the right upper and both the lower limbs remained. During the 4 months between the spinal surgery and his death, there was no further motor deterioration in any of his four extremities.
Conclusion:This case demonstrates the need to be aware of potential ISM in the follow-up of patients with ONB. The early detection of ISM by spinal MRI is crucial to ensuring good palliative care.
Keywords: Myelopathy, olfactory neuroblastoma, spinal metastasis, surgery
INTRODUCTION
Olfactory neuroblastoma (ONB) is a rare malignant intranasal neoplasm that originates from the neural crest cells of the olfactory epithelium.[
CASE REPORT
A 52-year-old man complained of nasal stuffiness along with bleeding, headache, and vomiting. He was referred to our department after magnetic resonance imaging (MRI) showed an enormous mass occupying the nasal and paranasal cavities and extending into the bilateral frontal base [Figure
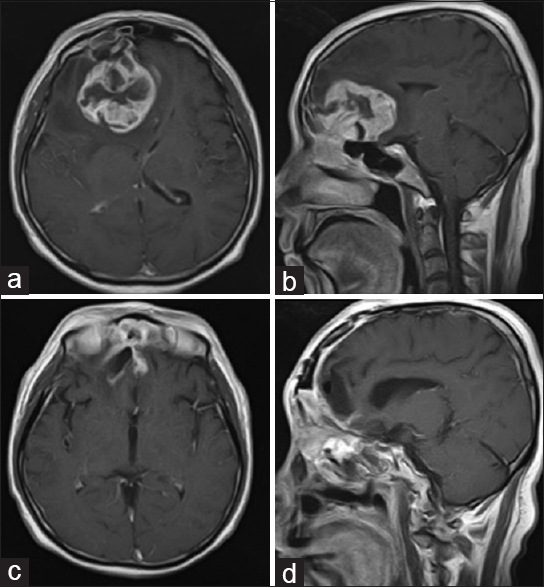
Figure 1
Axial (a) and sagittal (b) T1-weighted Gd-enhanced magnetic resonance imaging (MRI) on admission, showing a contrast-enhancing sinonasal mass with intracranial extension through the cribriform plate into the anterior cranial fossa. Postoperative axial (c) and sagittal (d) T1-weighted Gd-enhanced MRI demonstrating complete removal of the sinonasal and intracranial tumor
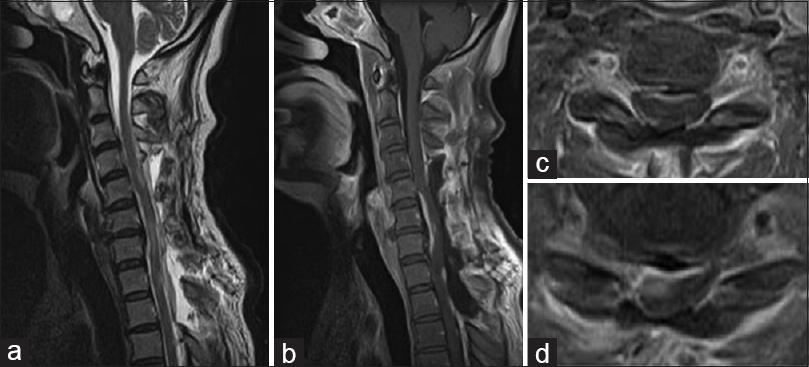
At 24 weeks postoperatively, the patient noted numbness in both upper limbs, which gradually worsened. He was readmitted 25 weeks after the surgery because of rapidly deteriorating symptoms. Neurological findings on readmission revealed tetraparesis, hypesthesia, and hypoalgesia below C6, hyperreflexia of both legs, and urinary incontinence. CT and MRI revealed an intradural lesion compressing the spinal cord along its right ventral aspect at C5 through T2 [Figure
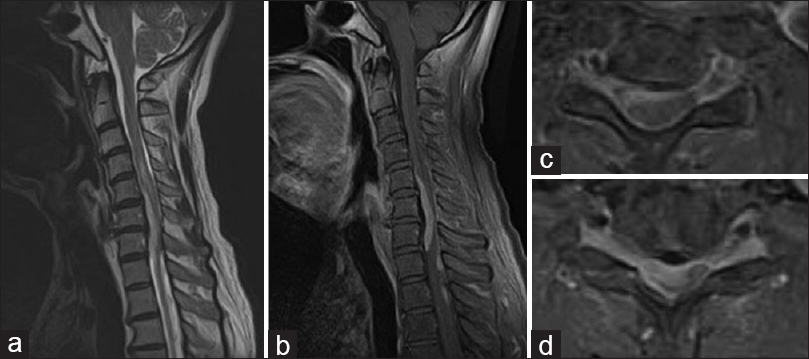
Figure 2
Magnetic resonance imaging on second admission. The T2-weighted images (WI) (a) and Gd-enhanced T1-WI (b) sagittal images show multiple intradural lesions between C3 and Th4. Gd-enhanced axial T1-WI images reveal compression of the spinal cord along its right ventral aspect at C4/5 (c) and C5/6 (d)
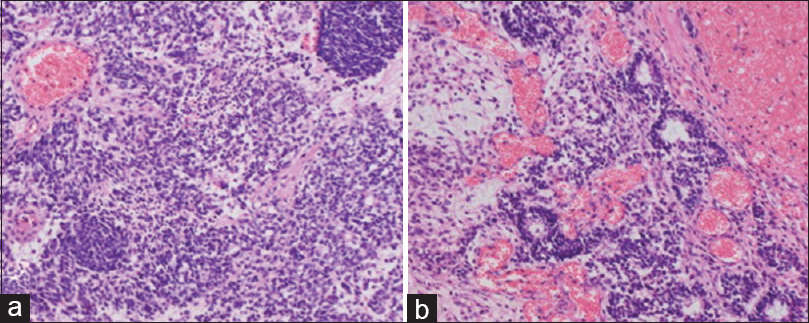
Figure 3
Photomicrographs of the spinal tumor. The hematoxylin and eosin-stained sections show tumor cells predominantly arranged in a densely growing pattern, scattered necrotic changes, nuclear fission, and Homer–Wright rosettes (a: ×100, b: ×200). These findings are consistent with Hyams grade 3 olfactory neuroblastoma and with the pathological findings of the first surgery
DISCUSSION
Approximately 2% of all sinonasal tract tumors are ONBs, corresponding to an incidence of approximately 0.4 per million population.[
Complete surgical resection of the tumor followed by radiation therapy is currently recognized as the optimal treatment for ONB. Although an effective routine therapeutic regimen has not been established,[
Motor weakness did not progress in our patient between spinal surgery and his death. However, ISM was not detected until it caused neurological symptoms. Thus, microsurgical resection of ISM should be considered in selected cases. Patients with rapidly worsening neurological deficits, no evidence of widespread organ metastasis, and a life expectancy of at least a few months should be considered for tumor resection.
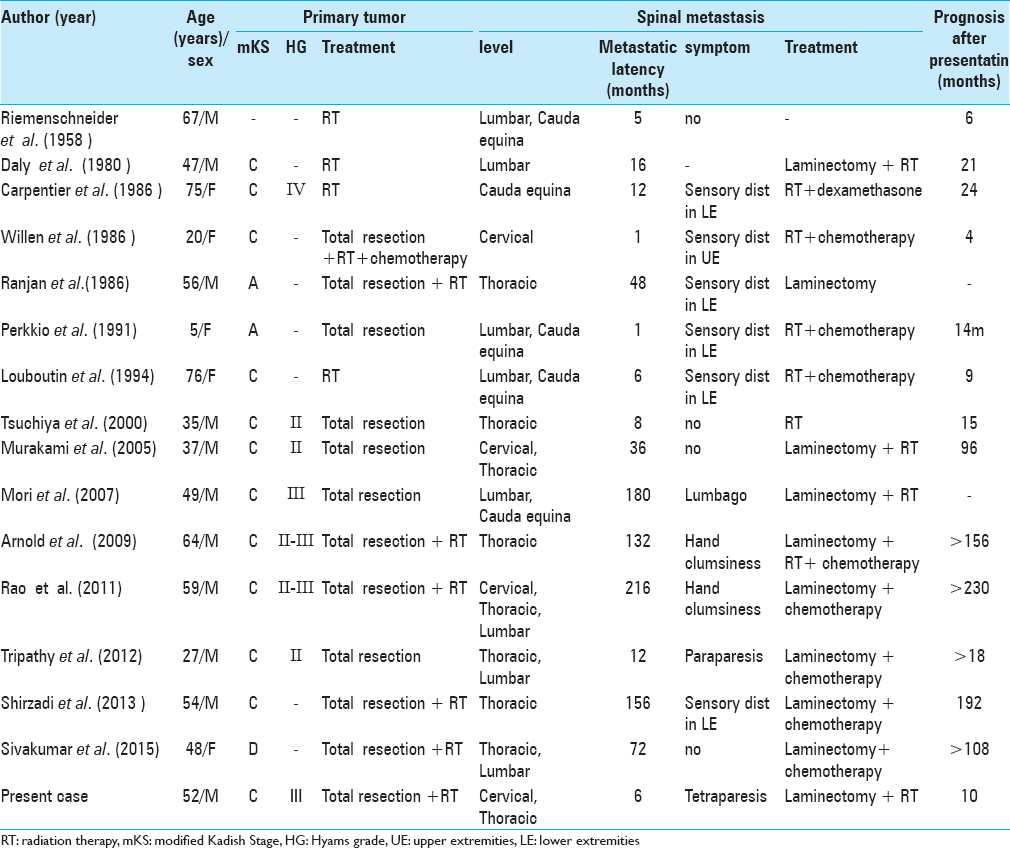
Although ONB is a rare malignant tumor, 15 cases of associated ISM have been reported [
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arnold PM, Habib A, Newell K, Anderson KK. Esthesioneuroblastoma metastatic to the thoracic intradural and extradural space. Spine J. 2009. 9: e1-5
2. Bradley PJ, Jones NS, Robertson I. Diagnosis and management of esthesioneuroblastoma. Curr Opin Otolaryngol Head Neck Surg. 2003. 11: 112-8
3. Broich G, Pagliari A, Ottaviani F. Esthesioneuroblastoma: A general review of the cases published since the discovery of the tumour in 1924. Anticancer Res. 1997. 17: 2683-706
4. Carpentier PJ, Ebels EJ. Olfactory neuroblastoma with spinal metastasis: A problem in diagnosis. Clin Neurol Neurosurg. 1986. 88: 203-8
5. Daly NJ, Voigt JJ, Combes PF. Diagnosis and treatment of olfactory neuroblastomas in seven patients. Int J Radiat Oncol Biol Phys. 1980. 6: 1735-8
6. Hyams VJ, Batsakis JG, Michaels L.editorsTumors of the upper respiratory tract and ear. Armed Forces Institute of Pathology Fascicles, 2nd series. Washington: American Registry of Pathology Press; 1988. p.
7. Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: A population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007. 133: 276-80
8. Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976. 37: 1571-6
9. Louboutin JP, Maugard-Louboutin C, Fumoleau P. Leptomeningeal infiltration in esthesioneuroblastoma: Report of two cases with poor prognosis. Eur Neurol. 1994. 34: 236-8
10. Lund VJ, Howard D, Wei W, Spittle M. Olfactory neuroblastoma: Past, present, and future?. Laryngoscope. 2003. 113: 502-7
11. Malouf GG, Casiraghi O, Deutsch E, Guigay J, Temam S, Bourhis J. Low- and high-grade esthesioneuroblastomas display a distinct natural history and outcome. Eur J Cancer. 2013. 49: 1324-34
12. Mori R, Sakai H, Kato M, Hida T, Nakajima M, Fukuda T. Olfactory neuroblastoma with spinal metastasis: Case report. No Shinkei Geka. 2007. 35: 503-8
13. Morita A, Ebersold MJ, Olsen KD, Foote RL, Lewis JE, Quast LM. Esthesioneuroblastoma: Prognosis and management. Neurosurgery. 1993. 32: 706-14
14. Murakami M, Kakita K, Kimura S, Hosokawa Y. Subdural extension of recurrent olfactory neuroblastoma. Case report. Neurol Med Chir. 2005. 45: 596-9
15. Perkkiö M, Majuri S, Hänninen A, Paljärvi L. A child with esthesioneuroblastoma with metastases to the spinal cord and the bone marrow. Med Pediatr Oncol. 1991. 19: 66-9
16. Ranjan D, Hennessy RG. Esthesioneuroblastoma: Cerebral and spinal metastases without direct cranial invasion. J Neurooncol. 1986. 4: 71-4
17. Rao AJ, Gultekin SH, Neuwelt EA, Cintrón-Colón HR, Ragel BT. Late occurrence of drop metastasis to the spine in a case of esthesioneuroblastoma. J Neurosurg Spine. 2011. 15: 571-5
18. Riemenschneider PA, Prior JT. Neuroblastoma originating from olfactory epithelium (esthesioneuroblastoma). Am J Roentgenol Radium Ther Nucl Med. 1958. 80: 759-65
19. Shaari CM, Catalano PJ, Sen C, Post K. Central nervous system metastases from esthesioneuroblastoma. Otolaryngology. 1996. 114: 808-12
20. Shetty SC, Gupta S, Chary G, Shariff S, Acharya V, Belliappa MS. Olfactory neuroblastoma metastatic to the breast. Rhinology. 2000. 38: 144-6
21. Shirzadi AS, Drazin DG, Strickland AS, Bannykh SI, Johnson JP. Vertebral column metastases from an esthesioneuroblastoma: Chemotherapy, radiation, and resection for recurrence with 15-year follow up. Case Rep Surg 2013. 2013. p. 107315-
22. Sivakumar W, Oh N, Cutler A, Colman H. Cranial and spinal leptomeningeal dissemination in esthesioneuroblastoma: Two reports of distant central nervous system metastasis and rationale for treatment. Surg Neurol Int. 2015. 25: S628-32
23. Tripathy P, Mohapatra D, Sarangi GS, Mohanty S. Esthesioneuroblastoma with early drop metastasis to spinal cord. Neurol India. 2012. 60: 436-7
24. Tsuchiya D, Kayama T, Kuchiki H, Sato S, Saito S. A case of olfactory neuroblastoma with intracranial extension and distant metastasis. No To Shinkei. 2000. 52: 811-6
25. Wade PM, Smith RE, John ME. Response of esthesioneuroblastoma to chemotherapy. Cancer. 1984. 53: 1036-41
26. Willen MA, Spiers AS, Hussain M. Esthesioneuroblastoma: Cerebrospinal fluid rhinorrhea and widespread metastasis. J Otolaryngol. 1986. 15: 80-4