- Department of Clinical Psychology, Na Homolce Hospital, Prague, Czech Republic.
- Department of Neurology, Epilepsy Center, Na Homolce Hospital, Prague, Czech Republic.
- Department of Neurosurgery, Na Homolce Hospital, Prague, Czech Republic.
- Department of Neurosurgery, University Hospital, Hradec Kralove, Czech Republic.
Correspondence Address:
Lenka Krámská, Department of Clinical Psychology, Epilepsy Center, Na Homolce Hospital, Prague, Czech Republic.
DOI:10.25259/SNI_335_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lenka Krámská1,2, Jan Šroubek3,4, Tomáš Česák4, Zdeněk Vojtěch2. One-year neuropsychological outcome after temporal lobe epilepsy surgery in large Czech sample: Search for factors contributing to memory decline. 17-Jun-2022;13:248
How to cite this URL: Lenka Krámská1,2, Jan Šroubek3,4, Tomáš Česák4, Zdeněk Vojtěch2. One-year neuropsychological outcome after temporal lobe epilepsy surgery in large Czech sample: Search for factors contributing to memory decline. 17-Jun-2022;13:248. Available from: https://surgicalneurologyint.com/surgicalint-articles/one-year-neuropsychological-outcome-after-temporal-lobe-epilepsy-surgery-in-large-czech-sample-search-for-factors-contributing-to-memory-decline/
Abstract
Background: Assessment of cognitive functions is an integral part of the evaluation the efficacy of temporal resections. We studied postoperative neuropsychological changes and factors contributing to worse memory outcomes in patients who experienced a significant decline using reliable change indices.
Methods: We prospectively studied 110 patients in whom we indicated anteromesial temporal resection (AMTR) and 46 patients who underwent selective amygdalohippocampectomy (SAHE). We administrated Wechsler Adult Intelligence Scale-Revised, Wechsler Memory Scale-Revised, and the Verbal Fluency Test before and 1 year after the operation.
Results: At a group level, we did not observe any statistically significant changes in global, verbal, and visual MQ in either the AMTR or the SAHE group. At an individual level, we found a mean decrease of verbal MQ after left-sided AMTR by −4.43 points (P = 0.01). We detected no significant differences between the left and right side of surgery in the SAHE group. In patients with significant postoperative memory decline, we found either pre-existing extrahippocampal deficits/postoperative complications or incomplete hippocampal resection or a combination of these factors.
Conclusion: In addition to the side of surgery, structural integrity and functional adequacy of resected hippocampus and volume of resected tissue and preoperative extrahippocampal lesions/postoperative complications also contribute to postoperative memory decline after temporal lobe epilepsy surgery.
Keywords: Amygdalohippocampectomy, Anteromesial temporal resection, Intelligence, Memory, Neuropsychology, Temporal lobe epilepsy
INTRODUCTION
Temporal lobe epilepsy (TLE) is often refractory to anti-seizure medication and is the most common type of partial epilepsy referred for epilepsy surgery.[
For many years, anterior temporal lobectomy (anteromesial temporal resection [AMTR]) and selective amygdalohippocampectomy (SAHE) have been the gold standard approach for the treatment of medically refractory TLE, commonly leading to favorable seizure and neuropsychological outcomes along with minimal postsurgical complications.[
However, concerns for postoperative memory impairment frequently limit the perceived surgical candidacy of some patients, especially if the planned operation involves the speech-dominant side. Indeed, impaired verbal memory has historically been reported after the left temporal resections.[
However, it is important to realize that pharmacoresistant TLE is also associated with progressive memory impairment even in nonsurgical patients. In fact, TLE patients develop long-term memory dysfunction (especially involving declarative memory) early in life. While neuropsychological test performance may predict patients’ academic accomplishments and every day function, subjective memory complaints are also often related to psychiatric comorbidities such as depression, which complicates analysis.[
Thus, postoperative neuropsychological outcomes significantly depend on many factors, including the patient’s innate characteristics. Previously published studies preoperatively assess the risk of postoperative memory impairment according to (1) preoperative memory function and (2) morphological findings on the hippocampus.
There are several examples of the role of preoperative memory function. For instance, a larger memory deficit can be expected in preoperatively cognitively normal patients than in patients with preoperatively impaired memory abilities.[
The concept of structural integrity and functional adequacy of resected hippocampus is a leading theory on which preoperative patients’ counseling is based. In other words, patients with preoperatively morphologically intact hippocampus and preserved memory are at higher risk of postoperative memory decline than those with severely atrophic and sclerotic hippocampus and impaired memory.[
Another factor influencing postoperative cognitive outcomes is the extent and location of the resection, for example, whether or not the parahippocampal gyrus and entorhinal cortex are targeted.[
Some studies have reported statistically lower freedom from recurrent seizures after SAHE compared to AMTR.[
The aim of our descriptive and exploratory study is as follows: (1) to compare the cognitive performance before and 1 year after AMTR and SAHE in a large sample of patients from a tertiary epilepsy center (observation period from 2000 to 2019) and (2) to concentrate on those patients in which memory significantly worsened (using RCI) after the operation and explore additional factors contributing to this deterioration.
MATERIALS AND METHODS
Patient selection
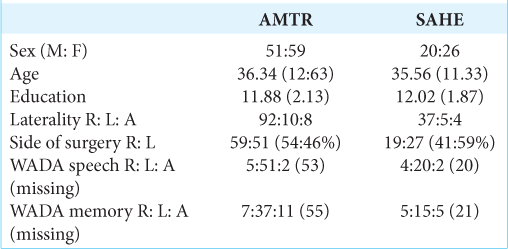
We studied a large sample of consecutive patients who underwent AMTR (n = 110) and 46 patients who underwent SAHE at the tertiary Epilepsy center at Na Homolce Hospital, Prague, the Czech Republic, during the period from 2000 to 2019. Only those who had been neuropsychologically evaluated and followed at our center were included in the study. Some patients did not complete the entire battery of cognitive tests (sensory, movement or verbal deficits before or postsurgery, insufficient effort, and poor cooperation during neuropsychological assessment).
Demographic data are summarized in
After the comprehensive preoperative evaluation, the patients were educated about their treatment options. SAHE was preferred whenever the operation involved the left and dominant hemisphere in the right-handed individuals.
Surgical technique
AMTR includes resection of the anterior part of temporal neocortex, amygdala, uncus, parahippocampal gyrus, and hippocampus proper. The resection of the anterior temporal neocortex spares a part of the superior temporal gyrus and is 3–4 cm in length on the dominant side and 4–5 cm on the nondominant side according to Spencer.[
The SAHE is performed according to Niemeyer[
To diminish any possible vascular injury, several steps are followed. In general, the resection is subpial, hence diminishing the probability of injury to Sylvian vessels and to vessels in an optocarotid, ambient, and crural cisterns. Larger cortical temporal arteries that traverse through the resected area to the dorsal temporal neocortex are, if possible, separated and preserved along with any larger draining veins. Any difficulties in finding of temporal horn (in the case of SAHE) are easily overcome using perioperative ultrasound; this modality is also used to check the extent of resection and to reveal any possible hemorrhage.
Neuropsychological evaluation
All patients were evaluated preoperatively and 1 year (2- and 5-year follow-ups are not included in this study) after surgery with a comprehensive neuropsychological battery. They were tested during 2 consecutive days; each session took 90–120 min, on average. During the first session, a psychological interview was performed and the Wechsler Adult Intelligence Scale-Revised (WAIS-R) was administered. Memory and verbal functions were assessed using the Wechsler Memory Scale-Revised (WMS-R)[
Statistical analysis
All values are presented as mean and standard deviation (SD) for continuous variables and as the number (percent) of subjects for categorical variables. Wilcoxon t-test was used to evaluate differences before and after the surgery and Mann–Whitney tests for differences in cognitive performance between AMTR and SAHE group at baseline and postoperatively (as a change from baseline). We report P-values that were uncorrected for multiple comparisons. The effect of age on memory functioning was evaluated using Pearson correlation. Statistical analyses were performed using R (R Core Team, 2020).[
We estimated the surgery effect at an individual level and employed the RCI classification.[
RESULTS
Surgical results
After AMTR, 68% of patients were classified as Engel I, 13% as Engel II, 12% as Engel III, and 7% as Engel IV. After SAHE, comparable seizure outcomes were found. Engel Class I had 70%, Engel II 15%, Engel III 13%, and Engel IV 2% of patients [
The proportion of seizure-free (IA and IB) and nonseizure-free (IC to IV) patients was comparable for both approaches (χ2 (1) = 0.07, P = 0.792).
Neuropsychological outcomes after AMTR
Intellectual outcomes
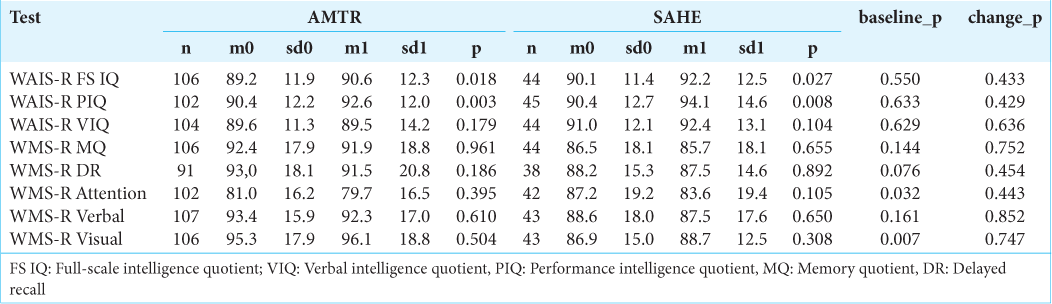
At the group level, we detected a statistically significant increase in Global and Performance IQ (P = 0.018, and P = 0.003, respectively) [
Memory outcomes
At a group level, we did not observe any statistically significant changes in global, verbal, and visual MQ, attention, and delayed recall (P = 0.961; 0.610; 0.504; 0.395; and 0.186, respectively).
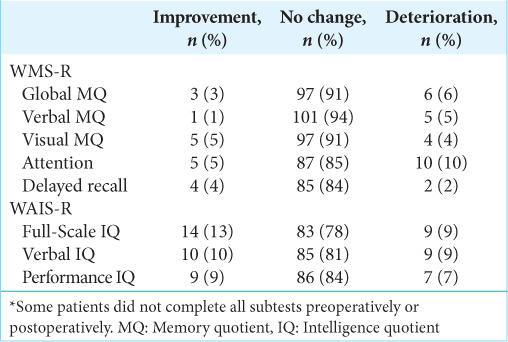
Using RCI [
When the relationship between age and memory performance postsurgery was examined using Pearson correlation, we detected a trend toward negative correlation in almost all measures, although this failed to reach statistical significance: WMS MQ (r = −0.4, P = 0.67, n = 105), WMS verbal MQ (r = −0.7, P = 0.48, n = 106), and WMS visual MQ (r = 0.09, P = 0.32, n = 105).
Comparison of cognitive performance according to the side of AMTR
Using a (unpaired) Mann–Whitney U-test, we compared changes in pre- and postoperative performance according to the side of AMTR for all measures. We detected significant difference between the left and right side of surgery only in verbal MQ. Mean decrease of verbal MQ after the left-sided surgery was −4.43 points; mean increase of verbal MQ after the right-sided surgery was 1.81 points, (P = 0.013). Differences in all other measures were not statistically significant.
Neuropsychological outcomes after SAHE
Intellectual outcomes
In the whole patient cohort, we observed a small, but significant improvement in global and performance IQ (P = 0.027 and P = 0.008, respectively) after SAHE [
Memory outcomes
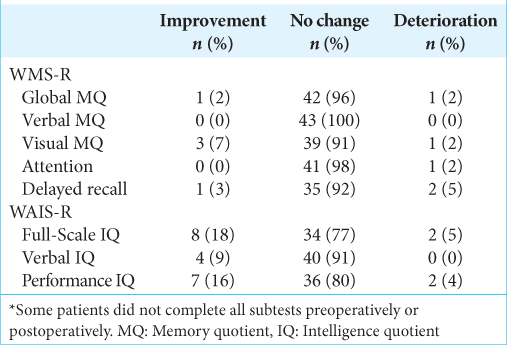
At the group level, we did not detect any statistically significant changes in global, verbal, and visual MQ, attention, and delayed recall (P = 0.655; 0.650; 0.308; 0.105; and 0.892, respectively) after SAHE. Using RCI at the individual level [
Negative but nonsignificant Pearson correlations between age and memory performance in all measures postsurgery were found: WMS MQ (r= −0.15, P = 0.35, n=41), WMS verbal MQ (r = −0.18, P = 0.24, n = 41), and WMS visual MQ (r = −0.02, P = 0.9, n = 41).
Comparison of cognitive performance according to the side of SAHE
Using (unpaired) Mann–Whitney U-test, we compared changes in pre- and postoperative performance according to the side of SAHE for all measures. We detected no significant differences between the left and right side of surgery.
Comparison of neuropsychological changes between AMTR and SAHE procedure
We compared mean postoperative change in all memory and intelligence quotients between AMTR and SAHE. We did not detect any significant differences in postoperative performance between these two procedures [
Data of patients in whom we found postoperative memory decline
[
DISCUSSION
This descriptive and exploratory study focuses on postoperative neurocognitive performance after AMTR and SAHE in a large sample of patients from a tertiary epilepsy center (observation period from 2000 to 2019). We also targeted on patients with significant memory decline postsurgery (using RCI) and explored epileptological and MRI findings contributing to this deterioration.
At the group level, we did not observe any statistically significant deterioration in memory and intelligence quotients measured by WAIS-R and WMS-R after AMTR and SAHE. Neuropsychological and epileptological results were satisfactory in our patients and comparable with previously published studies.[
We analyzed the possible causes of memory deterioration in both subgroups in addition to what those that have already been published. We detected two subgroups of patients with significant memory decline: (1) individuals with clinically manifested surgical complications or with large postoperative gliosis and (2) patients with residues of resected left hippocampus.
At the individual level, 6 (6%) patients deteriorated in global MQ, 5 (5%) in verbal MQ, 4 (4%) in visual MQ, 10 (10%) in attention, and 2 patients (2%) in delayed recall after AMTR.
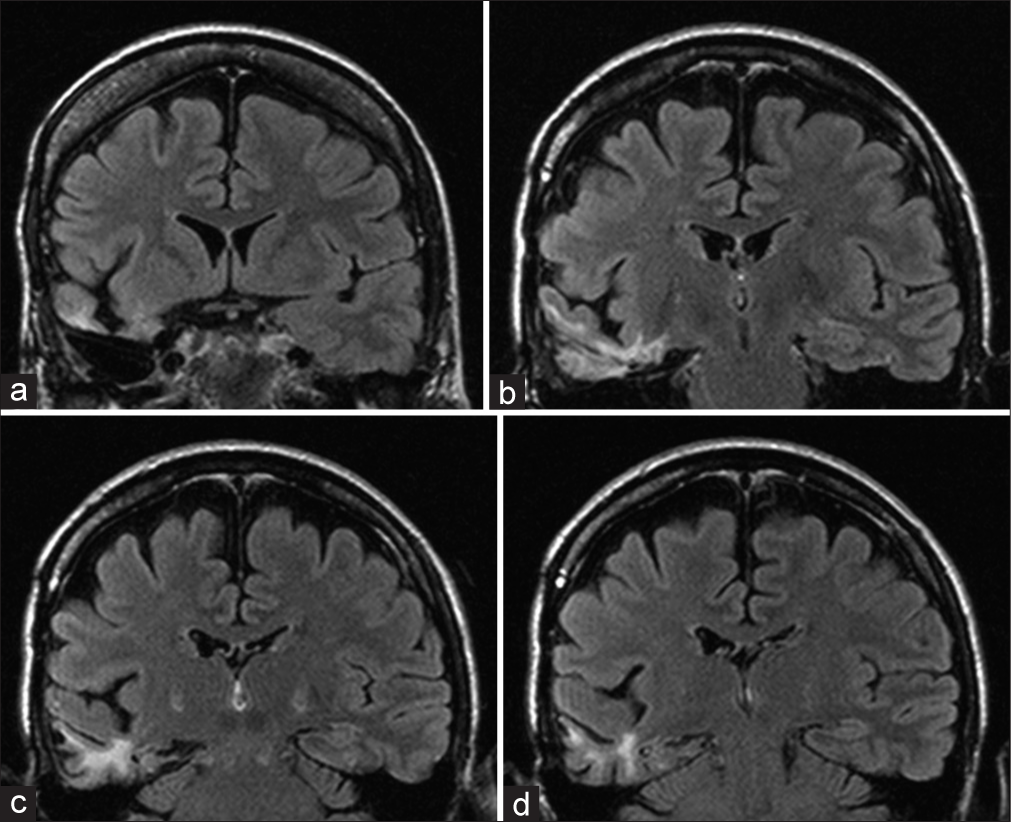
Those patients that deteriorated in global and verbal memory were operated on the left side (four patients) and on the right side (two patients). One patient suffered from moderate expressive aphasia, mild paresis of his right hand, and temporodorsal ischemia from resection, as confirmed by an MRI (Engel Class 1A). One patient suffered from transient phatic disorder and prosopagnosia (Engel Class 1A). Gliotic changes were detected in all patients. In one patient, there was ischemia, and in two patients, there was remnant hippocampus.
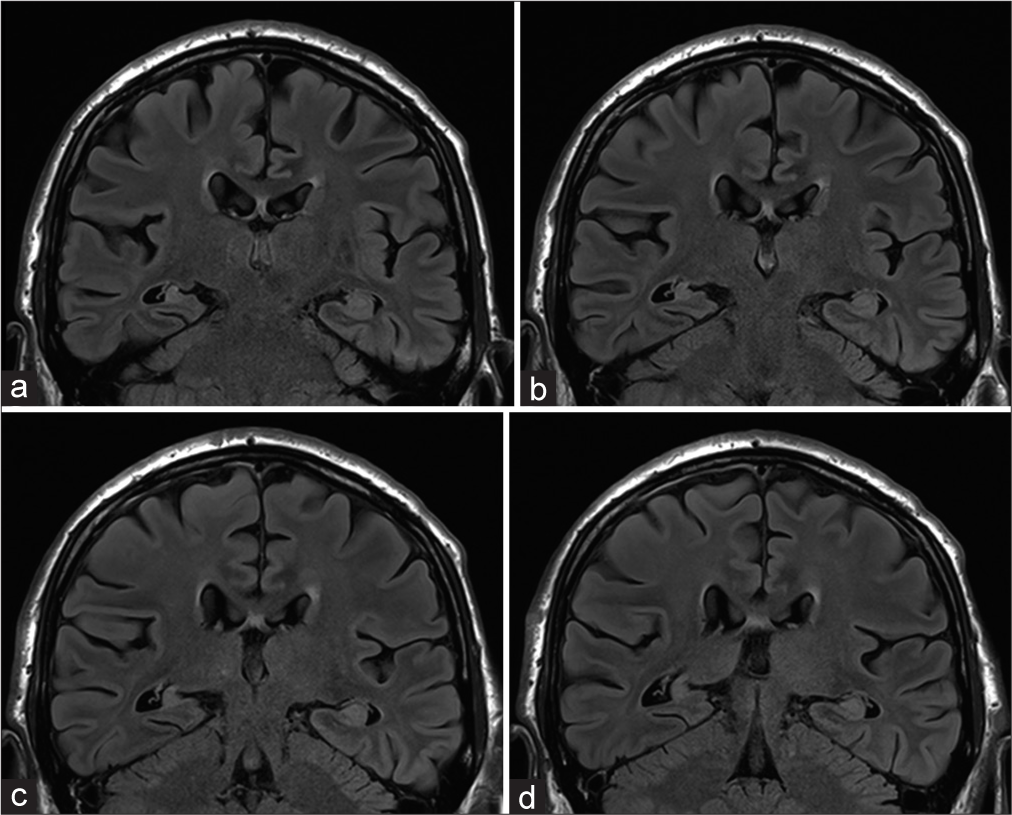
After SAHE, at the individual level, we detected deterioration in 1 (2%) patient in global MQ, 1 (2%) in visual MQ, and 1 (2%) attention. Two (5%) patients deteriorated in delayed recall. Two patients who deteriorated in memory performance were operated on the left side with the right hand dominance (Engel Class Ia and IIIa). One patient suffered from the right upper quadrantopsia and a residuum of the hippocampus was detected in two patients.
When we compared mean postoperative change in all memory and intelligence quotients between AMTR and SAHE, we did not detect any significant differences. Thus, from the neuropsychological point of view, SAHE may not have an advantage over AMTR due to the higher risk of complications and gliosis in the access pathway and beyond. It is debatable how residual hippocampi can affect cognitive function even in patients who are seizure free after surgery. It is conceivable that this is caused by the effect of hippocampal epileptiform activity on the residual hippocampus. In patients with pre-existing extrahippocampal brain lesions or surgical complications, memory decline after operation could be expected as larger volumes of the brain are damaged. The answer to why patients with incomplete resection of the posterior hippocampus tend to suffer from postoperative amnestic deficits is less clear. In our group of patients, we suspect that persistent seizures were not likely involved as their Engel Class is mostly favorable. We hypothesize that hippocampal remnants may still be able to produce disruption of brain functions, for example, by generating interictal epileptic discharges.[
In spite of the fact that AMTR involves resection of the lateral temporal cortex implicated in verbal information processing, several studies have not confirmed greater impairment in verbal memory tasks for this type of surgery compared to SAHE.[
The impairment of memory performance postsurgery has been in the center of attention for many years. With regard to the side of the surgery, temporal lobe resection (TLE) has a well-known effect on memory changes, especially on the verbal memory after the surgery in the functionally dominant hemisphere. Results of a meta-analysis demonstrated verbal memory decline in patients who underwent AMTR in dominant hemisphere.[
Even though our study is observational and exploratory (we did not have predefined hypotheses about individual outcomes or random assignment to treatments), we chose to retrospectively compare the outcomes results according to the side of surgery and reported uncorrected P-values. We compared changes in pre- and postoperative performance according to the side of SAHE for all measures. We detected no significant differences between the left and right side of surgery.
After AMTR, we detected a significant difference between the left and right side of surgery only in verbal MQ – with worse results after the left-sided surgery. Mean decrease of verbal MQ after the left-sided surgery was −4.43 points; mean increase of verbal MQ after the right-sided surgery was 1.81 points, (P = 0.013).
More SAHE patients (59%) were operated on the left side and AMTR resections were performed in 46% of patients on the left side. Selective approach was preferred in patients almost all operated in the left, dominant hemisphere, who were right handed and who had functionally dominant side of surgery.
Other intervening factors, such as preoperative cognitive function and age, also have a well-known influence on neuropsychological testing results after the TLE surgery. Greater postoperative memory impairment has been associated with older age at seizure onset and older age at the time of surgery.[
Lower general cognitive skills before surgery have also been described as a predictor of better verbal learning in patients after the right temporal lobe resection.[
The range of postoperative memory decline is also significantly affected by the presence of mesial temporal lobe pathology and the functional integrity of the resected mesial temporal lobe structures, as proven by preoperative memory assessment.[
Postoperative seizure freedom is associated with functional improvement in unresected areas contralaterally and ipsilaterally. The existence of an epileptogenic focus in the unilateral temporal lobe may be supported by severe hypometabolism restricted to the unilateral temporal lobe, with ipsilateral dominant hypometabolism.[
Besides seizures, TLE patients suffer from cognitive deficits that negatively influence everyday functioning and quality of life. Cognitive neuroscience traditionally researched TLE as an important model allowing for understanding human language and memory deficit as a result of hippocampal damage.[
Recently, our insight into the structural and functional pathology and cognitive profile in TLE has been enhanced by the advent of high-resolution and multimodal neuroimaging and by the increased use of comprehensive neurocognitive phenotyping batteries.[
Our study has several limitations
It is a single-center study There are a limited number of patients in each group We included different types of surgery procedures without random assignment to treatment We did not analyze patients without significant postoperative neurocognitive change in detail Not all patients completed the entire testing battery (sensory, movement, or verbal deficits before or postsurgery, insufficient effort, and cooperation during neuropsychological assessment) Lack of administration of new comprehensive neurocognitive phenotyping batteries.
CONCLUSION
Improvement in seizure and neuropsychological outcomes constitutes the essential aim of successful surgical treatment of pharmacoresistant MTLE.[
Based on our analysis, the majority of memory declined patients experienced clinically manifested surgical complications, large postoperative gliosis, or significant residues of resected left hippocampus. We are convinced that surgical complications are important intervening factors that significantly influence postsurgical memory performance and should be involved in the future neuropsychological outcome studies.
Our work should be the impetus for a multicenter study that would examine:
Not only the total volume of resected tissue but also quantification of gliotic tissue Cognitive results after resections of hippocampal residues.
Authors’ contributions
LK and ZV prepared the design of the study, participated in the acquisition, and analysis of data; LK wrote the original draft of the manuscript and critically revised the manuscript; and JS, TC, and ZV participated in the acquisition and analysis of data, critically revised and edited the manuscript. All the authors meet the standard criteria of authorship based on recommendations of the international committee of medical journal editors.
Ethical committee agreement
All data collection, storage, and processing were done in compliance with the Helsinki Declaration. All patients provided signed, informed consent and the studies IG 171501 and IG 193001 were approved by the ethics committee of Na Homolce Hospital in Prague.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The study was supported by the Ministry of Health of the Czech Republic, grant MH CZ and DRO NHH, IG 171501 and IG 193001.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank to Jakub Šroubek MD, Ph.D. and Lorna Myers. Ph.D. for English editing. We also thank to Dr. Jiří Lukavský for the statistical analysis.
References
1. Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984. 107: 293-308
2. Barr W. What happens to the brain following anterior temporal lobe resection?. Epilepsy Curr. 2016. 16: 316-8
3. Barr WB, Goldberg E, Wasserstein J, Novelly RA. Retrograde amnesia following unilateral temporal lobectomy. Neuropsychologia. 1990. 28: 243-55
4. Baxendale S. The impact of epilepsy surgery on cognition and behavior. Epilepsy Behav. 2008. 12: 592-9
5. Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011. 7: 154-64
6. Boucher O, Dagenais E, Bouthillier A, Nguyen DK, Rouleau I. Different effects of anterior temporal lobectomy and selective amygdalohippocampectomy on verbal memory performance of patients with epilepsy. Epilepsy Behav. 2015. 52: 230-5
7. Clusmann H, Schramm J, Kral T, Helmstaedter C, Ostertun B, Fimmers R. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002. 97: 1131-41
8. Foged MT, Vinter K, Stauning L, Kjær TW, Ozenne B, Beniczky S. Verbal learning and memory outcome in selective amygdalohippocampectomy versus temporal lobe resection in patients with hippocampal sclerosis. Epilepsy Behav. 2018. 79: 180-7
9. Helmstaedter C, Elger CE, Hufnagel A, Zentner J, Schramm J. Different effects of left anterior temporal lobectomy, selective amygdalohippocampectomy, and temporal cortical lesionectomy on verbal learning, memory, and recognition. J Epilepsy. 1996. 9: 39-45
10. Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003. 54: 425-32
11. Helmstaedter C, Roeske S, Kaaden S, Elger CE, Schramm J. Hippocampal resection length and memory outcome in selective epilepsy surgery. J Neurol Neurosurg Psychiatry. 2011. 82: 1375-81
12. Hermann BP, Wyler AR, Somes G, Berry AD, Dohan FC. Pathological status of the mesial temporal lobe predicts memory outcome from left anterior temporal lobectomy. Neurosurgery. 1992. 31: 652-6
13. Hoppe C, Elger CE, Helmstaedter C. Long-term memory impairment in patients with focal epilepsy. Epilepsia. 2007. 48: 26-9
14. Hu WH, Zhang C, Zhang K, Meng FG, Chen N, Zhang JG. Selective amygdalohippocampectomy versus anterior temporal lobectomy in the management of mesial temporal lobe epilepsy: A meta-analysis of comparative studies: A systematic review. J Neurosurg. 2013. 119: 1089-97
15. Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991. 59: 12-9
16. Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013. 80: 1669-76
17. Kleen JK, Scott RC, Holmes GL, Roberts DW, Rundle MM, Testorf M. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013. 81: 18-24
18. Liu A, Thesen T, Barr W, Morrison C, Dugan P, Wang X. Parahippocampal and entorhinal resection extent predicts verbal memory decline in an epilepsy surgery cohort. J Cogn Neurosci. 2017. 29: 869-80
19. Mansouri A, Fallah A, McAndrews MP, Cohn M, Mayor D, Andrade D. Neurocognitive and seizure outcomes of selective amygdalohippocampectomy versus anterior temporal lobectomy for mesial temporal lobe epilepsy. Epilepsy Res Treat. 2014. 2014: 306382
20. Matějček Z, Žlab Z.editors. Test Manual: Lateral Dominance Examination. Brno: Psychodiagnostika; 1972. p.
21. Milner B.editors. Memory and the medial temporal regions of the brain. Biology of Memory. New York: Academic Press; 1970. p.
22. Niemeyer P.editors. The Transventricular Amygdalohippocampectomy in Temporal Lobe Epilepsy, in Temporal Lobe Epilepsy. Illinois: CC Thomas Springfield; 1958. p. 461-82
23. Olivier A. Surgical techniques in temporal lobe epilepsy. Clin Neurosurg. 1997. 44: 211-41
24. Paglioli E, Palmini A, Portuguez M, Paglioli E, Azambuja N, da Costa JC. Seizure and memory outcome following temporal lobe surgery: Selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006. 104: 70-8
25. Language and Environment for Statistical Computing. Available from: https://www.R-project.org [Last accessed on 2022 Apr 09].
26. Rodríguez-Cruces R, Bernhardt BC, Concha L. Multidimensional associations between cognition and connectome organization in temporal lobe epilepsy. Neuroimage. 2020. 213: 116706
27. Saling MM. Verbal memory in mesial temporal lobe epilepsy: Beyond material specificity. Brain. 2009. 132: 570-82
28. Sauvigny T, Brückner K, Dührsen L, Heese O, Westphal M, Stodieck SR. Neuropsychological performance and seizure control after subsequent anteromesial temporal lobe resection following selective amygdalohippocampectomy. Epilepsia. 2016. 57: 1789-97
29. Schmeiser B, Wagner K, Schulze-Bonhage A, Mader I, Wendling AS, Steinhoff BJ. Surgical treatment of mesiotemporal lobe epilepsy: Which approach is favorable?. Neurosurgery. 2017. 81: 992-1004
30. Seidenberg M, Pulsipher DT, Hermann B. Cognitive progression in epilepsy. Neuropsychol Rev. 2007. 17: 445-54
31. Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B. Is the underlying cause of epilepsy a major prognostic factor for recurrence?. Neurology. 1998. 51: 1256-62
32. Sherman EM, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L. Neuropsychological outcomes after epilepsy surgery: Systematic review and pooled estimates: Cognitive Change after Epilepsy Surgery. Epilepsia. 2011. 52: 857-69
33. Spencer DD, Spencer SS, Mattson RH, Williamson PD, Novelly RA. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984. 15: 667-71
34. Takahashi M, Soma T, Kawai K, Koyama K, Ohtomo K, Momose T. Voxel-based comparison of preoperative FDGPET between mesial temporal lobe epilepsy patients with and without postoperative seizure-free outcomes. Ann Nucl Med. 2012. 26: 698-706
35. Téllez-Zenteno JF, Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012. 2012: 630853
36. Vossel KA, Ranasinghe KG, Beagle AJ, Mizuiri D, Honma SM, Dowling AF. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol. 2016. 80: 858-70
37. Wechsler D.editors. Manual for the Wechsler Memory Scale Revised. San Antonio, Texas: The Psychological Corporation; 1987. p.
38. Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res. 2013. 104: 94-104
39. Whiting AC, Chen T, Swanson KI, Walker CT, Godzik J, Catapano JS. Seizure and neuropsychological outcomes in a large series of selective amygdalohippocampectomies with a minimally invasive subtemporal approach. J Neurosurg. 2020. 134: 1685-93
40. Wieser HG, Yaşargil MG. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol. 1982. 17: 445-57
41. Yang PF, Zhang HJ, Pei JS, Lin Q, Mei Z, Chen ZQ. Neuropsychological outcomes of subtemporal selective amygdalohippocampectomy via a small craniotomy. J Neurosurg. 2016. 125: 67-74
42. Yue J, Zhang CQ, Hou Z, Yang H. Subtemporal selective amygdalohippocampectomy in patients with mesial temporal lobe epilepsy: Systematic review of seizure and neuropsychological outcomes. Epilepsy Behav. 2020. 112: 107435