- Department of Neurosurgery, Hôpital des Spécialités, Rue Lamfadel Cherkaoui Rabat Institut, Rabat, Morocco,
- Department of Neurosurgery, Cheikh Khalifa Hospital, Ave Mohamed Taieb Naciri, Casablanca, Morocco,
- Anatomopathology Center, CAPA, Rabat, Morocco.
Correspondence Address:
Hajar Bechri, Department of Neurosurgery, Hôpital des Spécialités, Rue Lamfadel Cherkaoui Rabat Institut, Rabat, Morocco.
DOI:10.25259/SNI_613_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hajar Bechri1, Mohammed Yassaad Oudrhiri1, Sidi Mamoun Louraoui2, Adyl Melhaoui1, Sanae Sefiani3, Yasser Arkha1, Abdessamad El Ouahabi1. Papillary tumor of the pineal region: Is stereotactic radiosurgery efficient for this rare entity?. 03-Aug-2021;12:386
How to cite this URL: Hajar Bechri1, Mohammed Yassaad Oudrhiri1, Sidi Mamoun Louraoui2, Adyl Melhaoui1, Sanae Sefiani3, Yasser Arkha1, Abdessamad El Ouahabi1. Papillary tumor of the pineal region: Is stereotactic radiosurgery efficient for this rare entity?. 03-Aug-2021;12:386. Available from: https://surgicalneurologyint.com/surgicalint-articles/11018/
Abstract
Background: Papillary tumors of the pineal region are rare neuroepithelial lesions that were described for the 1st time in the WHO 2007 classification. Management of such lesions remains controversial.
Case Description: We describe the case of a 26-year-old female who presented with intracranial hypertension syndrome secondary to a 1.9 cm3 lesion of the pineal region causing hydrocephalus. The patient benefited from an endoscopic third ventriculocisternostomy and a biopsy of her lesion in favor of a papillary tumor of the pineal region. After discussion of the surgical risks, the patient refused the surgical option and a stereotactic radiosurgery (SRS) was performed. She improved both clinically (allowing her to regain autonomy) and radiologically (reduction of 60% of tumor volume) at 1 year follow-up.
Conclusion: Because of the rarity of the lesion, literature is yet not able to find consensus concerning management of such lesion, but SRS has proven efficiency for these Grades II or III lesions with high recurrence rates. Therefore, it should be considered as a primary therapeutic option allowing good outcome with low risks for the patient.
Keywords: Papillary tumor, Pineal region, Stereotactic radiosurgery
INTRODUCTION
Papillary tumor of the pineal region (PTPR) is a neuroepithelial tumor defined as Grade II or III, depending on its malignancy and frequency of local recurrence.[
CASE REPORT
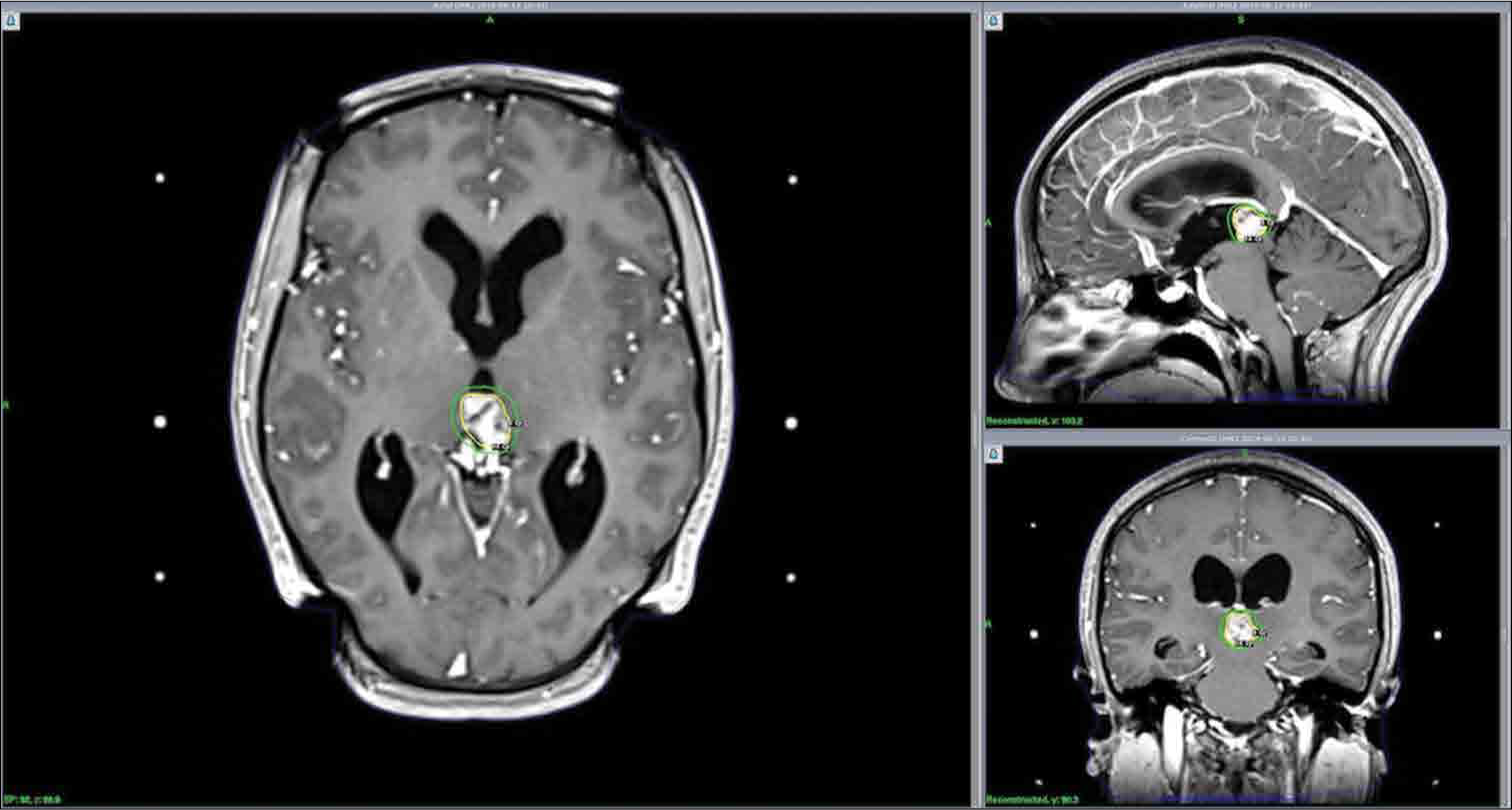
A 26-year-old female with no medical history presented with progressive headaches, vomiting, and visual loss over a 3-month period. The physical examination showed a Glasgow Coma Scale of 15, no motor or sensory deficits, and equal and semi-mydriatic pupils with only light perception in both eyes at the ophthalmological examination. The computed tomography (CT) scan and contrast magnetic resonance imaging (MRI) showed obstructive hydrocephalus secondary to a pineal region tumor. This lesion was hypointense on T1-weighted sequences and heterogeneously hyperintense on T2 with small cystic portions noted in the mass. Solid portions were heterogeneously enhanced after contrast injection. The tumor extended into the third ventricle, elevating both internal cerebral veins, but no extension into the fourth ventricle or the thalamus was noted. The lesion measured 15 mm × 10 mm. The serum laboratory workup for germ cell tumor markers (αfetoprotein and β-HCG) was negative [
Given the location of the lesion and its accessibility by transventricular biopsy, an endoscopic right ventricular approach was chosen both to treat the obstructive hydrocephalus endoscopic third ventriculostomy (ETV) and obtain tumor specimens for pathology. The postoperative protocol was determined based on the results of the first procedures. The transventricular biopsy was achieved through a two burr hole approaches: one in front of the coronal suture for the ETV (using the Decq Neuroendoscopy set [Karl Storz, Germany] with 30° endoscope, 2.9 mm in diameter and 30 cm in length, ventriculostomy forceps and a Fogarty balloon catheter) and the second, 5 cm in front of the coronal suture, to perform the biopsy using the same set and tumor forceps. The tumor was reddish and nonhemorrhagic, originating from the pineal region. Three tumor specimens were collected. A CSF sample was tested for pathological cells and biological markers and was negative for both.
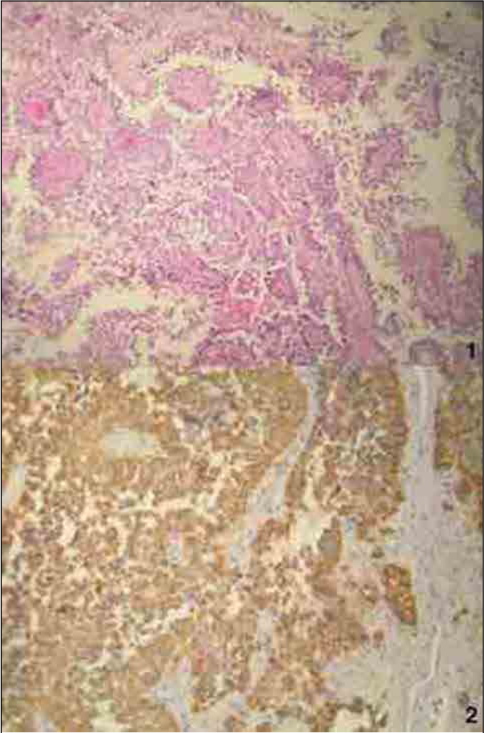
Pathology revealed an epithelial-looking tumor with papillary features and denser cellular areas in a vascularized conjunctive tissue. In papillary areas, the vessels were covered by layers of large, pale to eosinophilic columnar cells. The nuclei were round to oval, with stippled chromatin and pleomorphic nuclei. The mitotic count ranged from 0 to 10 mitoses per 10 high-power fields. Necrotic foci were noted. Vessels were hyalinized and had a pseudoangiomatous morphology, with multiple lumina and an absence of microvascular proliferation [
Figure 2:
Papillary tumor of the pineal region. (1) The vascular axes of neoplastic papillae often harbor multiple capillaries. Neoplastic cells detached from the papillary vascularized core, leading to an apparent clear perivascular space. (2) Cytokeratin AE1-AE3 is diffusely expressed in the epithelial-like neoplastic cells and predominates in perivascular areas.
The IHC profile showed reactivity to epithelial membrane antigen (EMA), synaptophysin, glial fibrillary acid protein (GFAP), vimentin, PS100, pancytokeratin, and Ki67 estimated at 2%, indicating a PTPR.
In the postoperative period, the patient showed significant improvement in intracranial hypertension syndrome symptoms (disappearance of headaches and vomiting), no motor or sensitive deficit, and a slight ophthalmological improvement at the examination (2/10 on both eyes).
Given the pathological findings in the tumor specimen, two treatment options were possible and explained in detail to the patient: surgical resection through one of the selected approaches or Gamma Knife-based SRS. The latter option was preferred, as the patient did not accept the risks associated with surgery and could be achieved 1 month after the biopsy.
The patient was fitted with a Leksell stereotactic frame (Model G, Elekta Instruments, AB Stockholm, Inc.) under local anesthesia. Gadolinium-enhanced T1-weighted reconstructions were generated and the treatment planning was performed using GammaPlan software (Elekta Instruments, Inc., version 11.0.3). The patient was treated 2 weeks after the biopsy for a tumor with a volume of 1.9 cm3. We delivered a 14 Gy dose at 50% isodose with 40 shots of 4 mm using a Leksell Gamma Knife ICON machine (Elekta Instruments, AB Stockholm, Inc.). The beam time was 88 min for a dose ratio of 2.681 Gy/min. The mean dose delivered to the brainstem was 0.4 ± 0.8; as such, the 10 Gy isodose volume was difficult to measurable. In the context of tumor location, the superior and inferior colliculi were initially considered as organs at risk; however, considering the mean dose to the brainstem, local dose distributions were not measurable. The frame was removed at the end of the procedure and the patient was discharged the same day.
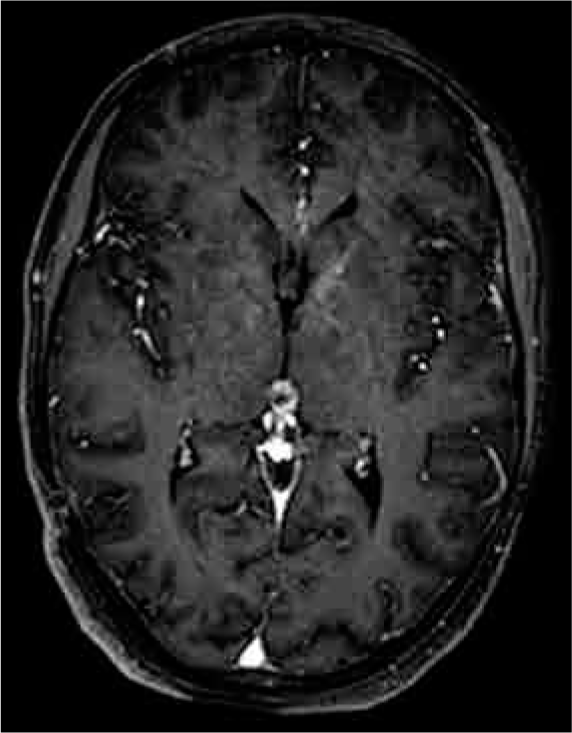
The patient follow-up occurred at 3, 6, and 12 months. Her neurological examination showed progressive improvement of the intracranial hypertension syndrome symptoms as well as her visual status. On MRI, we observed a significant tumor reduction at 6 and 12 months (30% and 60% volume reduction, respectively) and the previously reported aqueductal compression had disappeared at 6 months [
Because of the malignancy of the lesion and the high risk of recurrence, the patient will attend follow-up appointments every 6 months, including a physical examination and serial imaging. This will continue for the first 5 years, and yearly until the 10-year follow-up period is complete.
DISCUSSION
Tumors of the pineal region are rare and account for <1% of all brain tumors. Papillary tumors are the most recently identified pathological pineal region tumors, described by Jouvet et al. in 2003. The tumors originate from a distinct ependymal cell of the sub-commissural organ with epithelial-like growth patterns and, as described in our patient, fibrovascular papillae with a well-defined secretory function.[
Slight female predominance has been observed in the literature.[
Because of the rarity of the lesion, multiple therapeutic options have been described, but a standard treatment has yet to be determined. Surgical resection, mostly using an infratentorial supracerebellar approach, has been used as the primary therapeutic strategy to improve outcome, recurrence, and survival rates.[
The effectiveness of radiotherapy (craniospinal irradiation with a boost to the primary site, whole-brain radiotherapy with a boost to the primary site, focal irradiation of the pineal area only, or radiosurgery; each protocol depending on the center and the initial diagnosis) and chemotherapy (mainly based on cisplatinum-VP16 protocols) has been studied by Fauchon et al. who reported that there is no improvement in overall survival after these treatments.[
SRS is well known to be a safe alternative as a primary or adjuvant treatment for deeply located lesions. The first use of SRS for PTPR was in 2010 by Kim et al.[
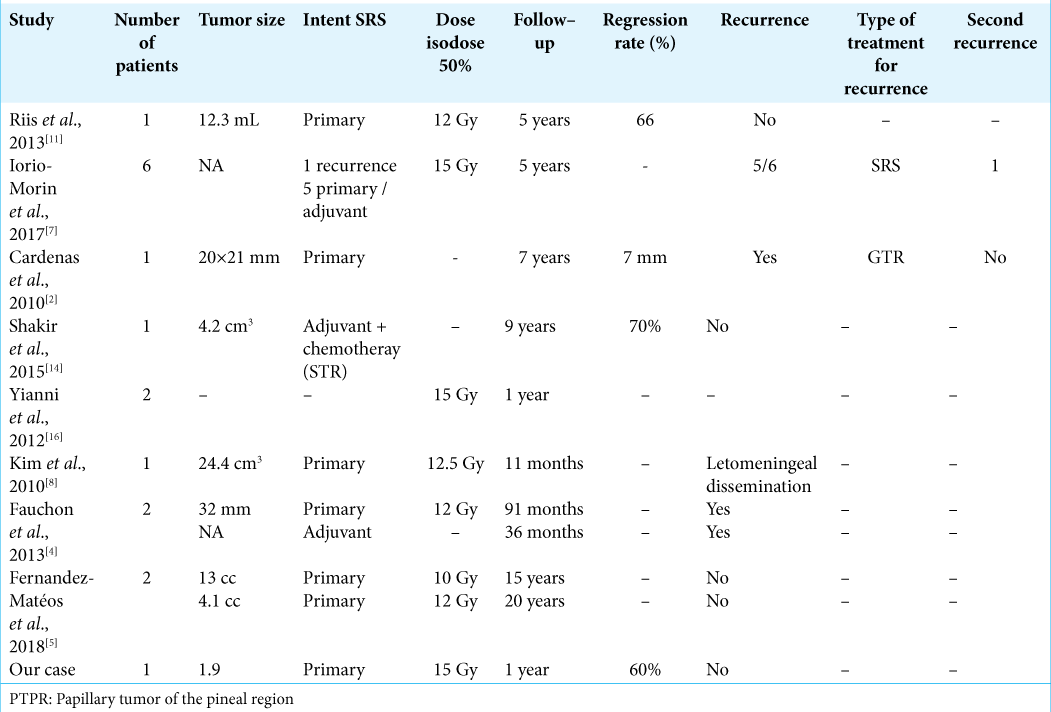
Iorio-Morin et al., in their report on SRS of pineal region tumors, considered SRS at tumor progression. They found 33% of local control at 5 years, with one patient experiencing a total disappearance of the tumor. They advocate for a second SRS treatment for recurrence or tumor progression.[
Although details about the treatment planning protocol used are not always available, all groups used a peripheral prescription dose of 10–15 Gy [
Unfortunately, there are limited cases of PTPR reported in the literature and, as such, a significant comparison between the different therapeutic modalities is not possible. Thus, the authors agree that prospective studies with larger cohorts are needed to determine the best therapeutic option to offer the patients. At present, the choice should be made by balancing the risks and benefits according to the surgeons’ technicity, the availability of the techniques, and the patients’ preference.[
CONCLUSION
PTPR are rare lesions with unpredictable courses. SRS may be considered as a primary or adjuvant treatment option due to its potential long-term local control local control of these surgically challenging lesions.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Cañizares Méndez ML, Delgado MA, Salgado JA, Ledezma JJ, Cabezuelo EC, Crespo FD. Papillary tumor of the pineal region: Case report and review of the literature. Neurocirugia (Astur: Engl Ed). 2019. 30: 38-43
2. Cardenas R, Javalkar V, Haydel J, Wadhwa R, Fowler M, Scheithauer B. Papillary tumor of pineal region: Prolonged control rate after gamma knife radiosurgery-a case report and review of literature. Neurol India. 2010. 58: 471-6
3. Edson MA, Fuller GN, Allen PK, Levine NB, Ghia AJ, Mahajan A. Outcomes after surgery and radiotherapy for papillary tumor of the pineal region. World Neurosurg. 2015. 84: 76-81
4. Fauchon F, Hasselblatt M, Jouvet A, Champier J, Popovic M, Kirollos R. Role of surgery, radiotherapy and chemotherapy in papillary tumors of the pineal region: A multicenter study. J Neurooncol. 2013. 112: 223-31
5. Fernández-Mateos C, Martinez R, Vaquero J. Long-term follow-up after radiosurgery of papillary tumor of pineal region: 2 Case reports and review of literature. World Neurosurg. 2018. 116: 190-3
6. Hua X, Yang P, Zhang M, Zhao Y, Wang B. Papillary tumor of the pineal region: A case report and review of the literature. Exp Ther Med. 2015. 10: 1375-9
7. Iorio-Morin C, Kano H, Huang M, Lunsford LD, Simonová G, Liscak R. Histology-stratified tumor control and patient survival after stereotactic radiosurgery for pineal region tumors: A report from the international gamma knife research foundation. World Neurosurg. 2017. 107: 974-82
8. Kim YH, Kim JW, Park CK, Kim DG, Sohn CH, Chang KH. Papillary tumor of pineal region presenting with leptomeningeal seeding. Neuropathology. 2010. 30: 654-60
9. Lancia A, Becherini C, Detti B, Bottero M, Baki M, Cancelli A. Radiotherapy for papillary tumor of the pineal region: A systematic review of the literature. Clin Neurol Neurosurg. 2020. 190: 105646
10. Mathieu D, Iorio-Morin C. Stereotactic radiosurgery for pineal region tumors. Prog Neurol Surg. 2019. 34: 173-83
11. Riis P, van Eck AT, Dunker H, Bergmann M, Börm W. Stereotactic radiosurgery of a papillary tumor of the pineal region: Case report and review of the literature. Stereotact Funct Neurosurg. 2013. 91: 186-9
12. Rosa M, da Rocha AJ, da Silva AZ, Rosemberg S. Papillary tumor of the pineal region: MR signal intensity correlated to histopathology. Case Rep Neurol Med. 2015. 2015: 315095
13. Rousseau A, Mokhtari K, Duyckaerts C. The 2007 WHO classification of tumors of the central nervous system-what has changed?. Curr Opin Neurol. 2008. 21: 720-7
14. Shakir HJ, Qiu J, Prasad D, Mechtler LL, Fenstermaker RA. Papillary tumor of the pineal region with extended clinical and radiologic follow-up. Surg Neurol Int. 2015. 6: S451-4
15. Yamaki VN, Solla DJ, Ribeiro RR, da Silva SA, Teixeira MJ, Figueiredo EG. Papillary tumor of the pineal region: Systematic review and analysis of prognostic factors. Neurosurgery. 2019. 85: E420-9
16. Yianni J, Rowe J, Khandanpour N, Nagy G, Hoggard N, Radatz M. Stereotactic radiosurgery for pineal tumours. Br J Neurosurg. 2012. 26: 361-6