- Department of Neurosurgery, NTT Medical Center Tokyo, Shinagawa, Tokyo,
- Department of Neurosurgery, Fuji Brain Institute and Hospital, Sugita, Fujinomiya, Shizuoka, Japan.
Correspondence Address:
Sho Tsunoda
Department of Neurosurgery, NTT Medical Center Tokyo, Shinagawa, Tokyo,
DOI:10.25259/SNI_47_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sho Tsunoda, Tomohiro Inoue, Hideaki Ono, Kazuaki Naemura, Atsuya Akabane. Paramedian thalamic infarction caused by cisternal drain placement in open clipping for aneurysmal subarachnoid hemorrhage: Two case reports. 27-Jun-2020;11:164
How to cite this URL: Sho Tsunoda, Tomohiro Inoue, Hideaki Ono, Kazuaki Naemura, Atsuya Akabane. Paramedian thalamic infarction caused by cisternal drain placement in open clipping for aneurysmal subarachnoid hemorrhage: Two case reports. 27-Jun-2020;11:164. Available from: https://surgicalneurologyint.com/surgicalint-articles/10111/
Abstract
Background: Some complications associated with cisternal drainage have been reported; however, there are few reports on direct vascular injury caused by cisternal drain. We experienced two rare cases of thalamic infarction caused by cisternal drain placement during open clipping for a ruptured anterior communicating artery (AcomA) aneurysm through an anterior interhemispheric approach.
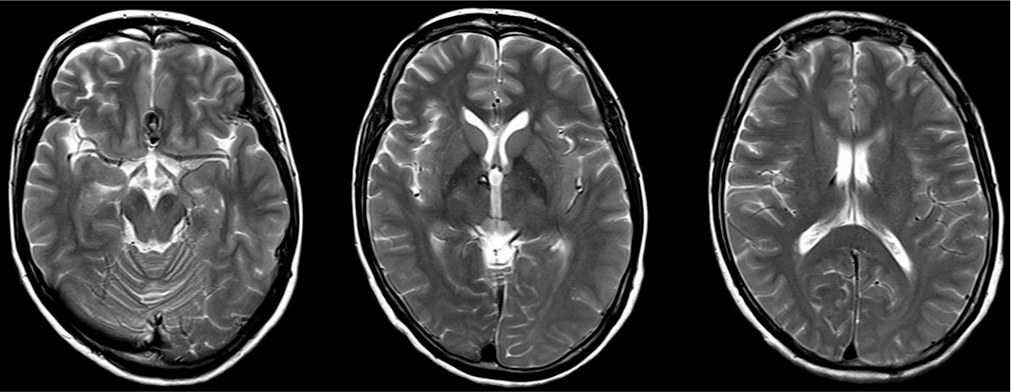
Case Description: Two cases of ruptured AcomA aneurysm were treated by surgical clipping through an anterior interhemispheric approach, and then a cisternal drain was inserted from opticocarotid space toward prepontine cistern. Postoperatively, the magnetic resonance imaging showed unilateral anterior-medial thalamic infarction in both two cases. By reviewing the postoperative computed tomography and digital subtraction angiography, it was suspected that the cisternal drain, which was inserted slightly deep, obstructed the P1 perforator because of an anatomical variation involving a lowered basilar bifurcation and caused postoperative unilateral paramedian thalamic infarction.
Conclusion: To avoid these complications, neurosurgeons should consider the potential for P1 perforator injury related to cisternal drain placement.
Keywords: Cisternal drainage, Complication, Subarachnoid hemorrhage, Thalamic infarction
BACKGROUND
Cerebral vasospasm often occurs in subarachnoid hemorrhage (SAH) patients secondary to a ruptured intracranial aneurysm and is a known prognostic factor for poor outcome. The spasmogenic substances released from the subarachnoid blood clots, and vascular hyperreactivity, are considered the leading causes of cerebral vasospasm.[
CLINICAL PRESENTATION
Case 1
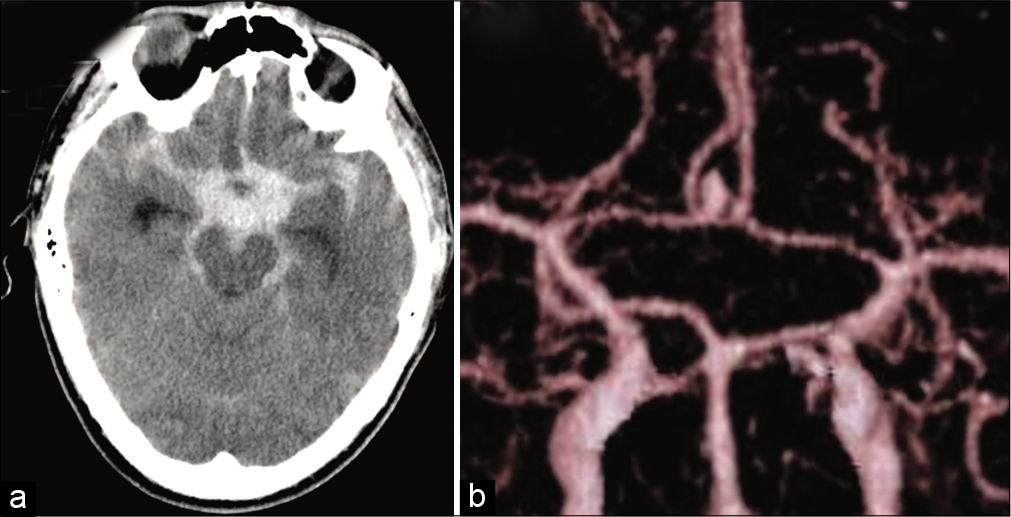
A 44-year-old man presenting with severe headache and disturbance in consciousness was admitted to our hospital with a Glasgow Coma Scale of 14 (E3V5M6), and no focal symptoms. Three-dimensional computed tomography (CT) scan revealed diffuse thickened SAH [
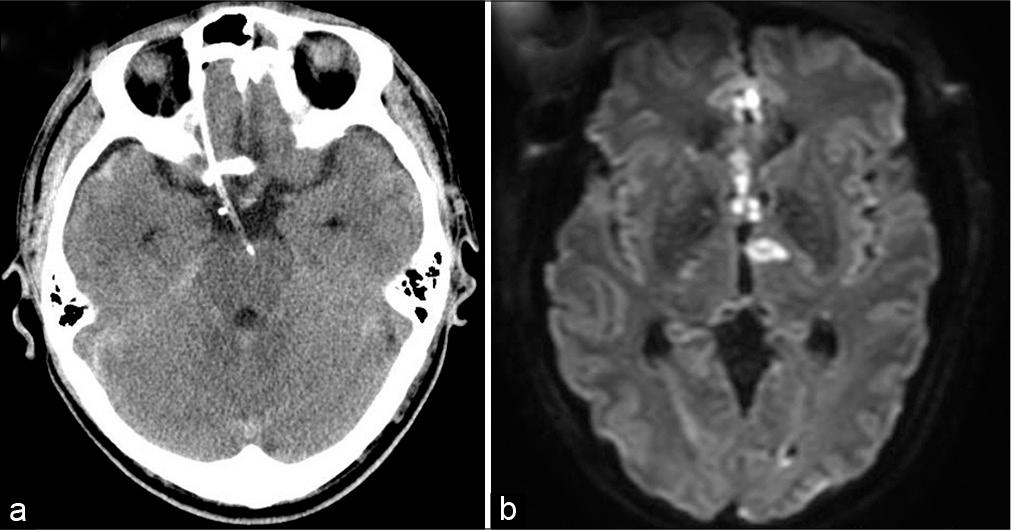
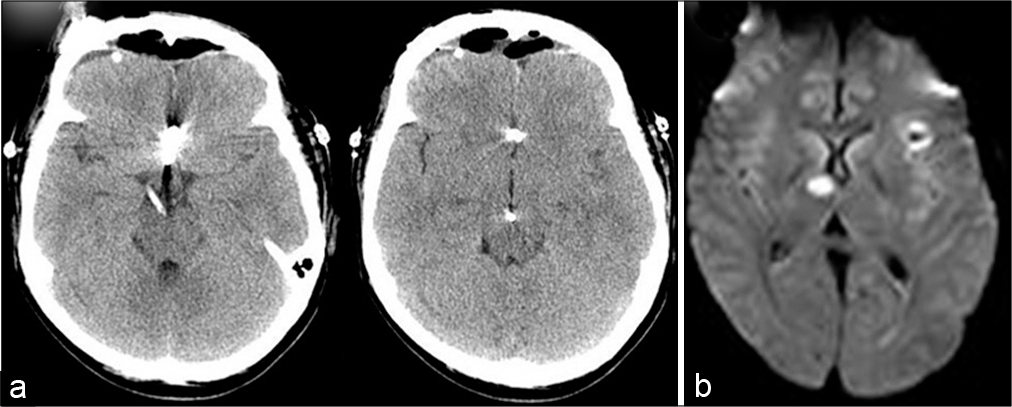
Postoperatively, CT scan demonstrated that tip of the cisternal drain was placed at the interpeduncular fossa [
Case 2
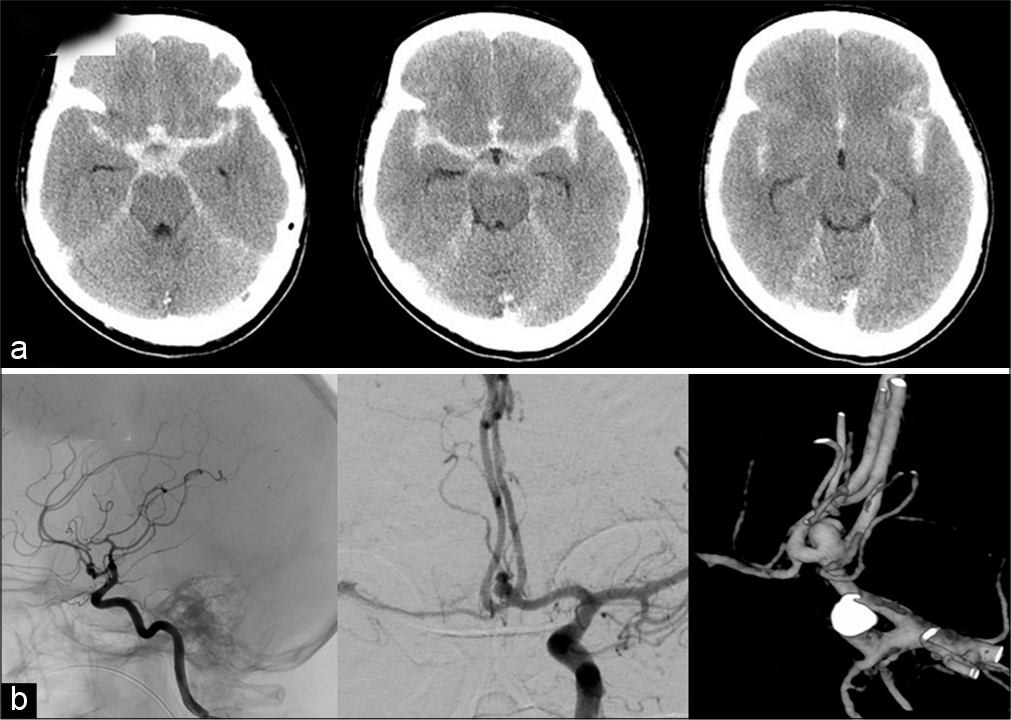
A 41-year-old women presented with WFNS Grade 2 SAH as same as Case 1. The image findings revealed diffuse SAH [
DISCUSSION
Cisternal drainage has great value in the management of SAH, despite the risks of meningitis and delayed hydrocephalus. Indeed, in our two cases, cisternal drainage allowed rapid removal of the subarachnoid clots. Nevertheless, our cases show the potential for direct vascular injury following cisternal drain placement. Horiuchi et al. reported medial branch injury of the basilar artery caused by cisternal drain placement.[
In our two cases, the sites of infarction involved the paramedian thalamic region, and we suspected perforator injury arising from the P1 segment of the posterior cerebral artery. Pedroza et al. classified P1 perforator injury into that involving the paramedian thalamic artery, superior paramedian mesencephalic artery, and inferior paramedian mesencephalic artery locations.[
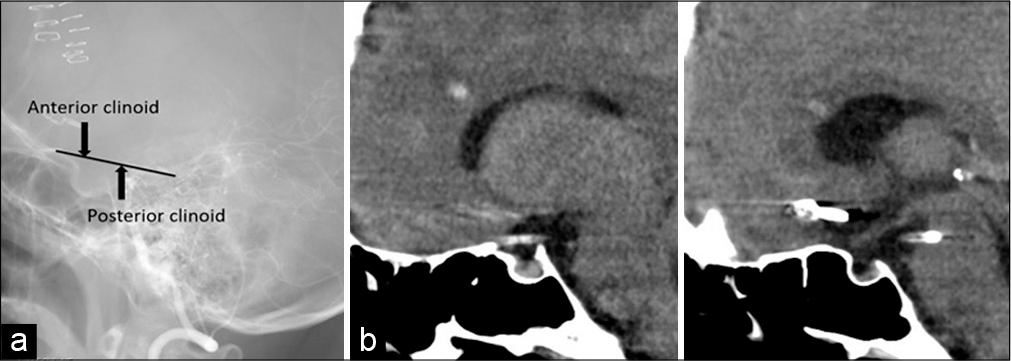
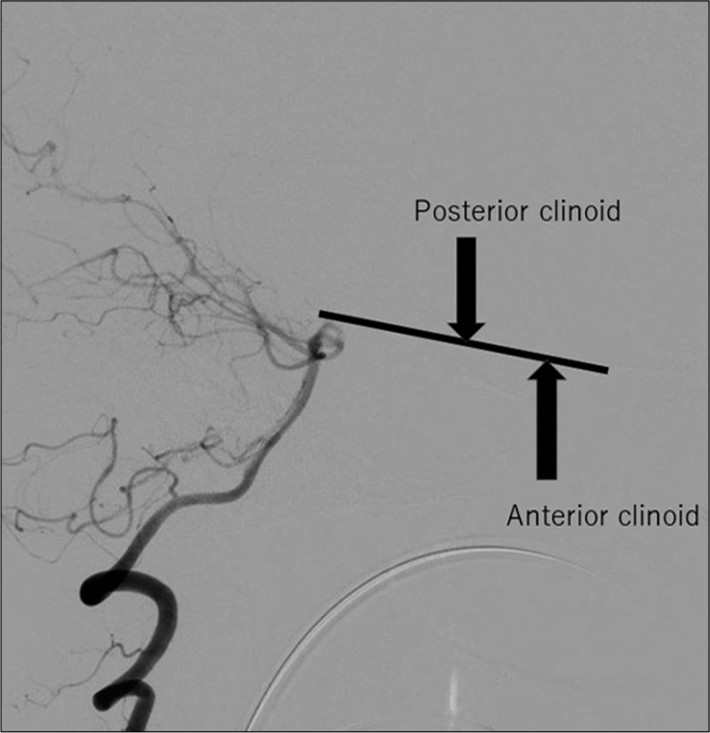
Cisternal drains are generally placed from the opticocarotid or carotidtentorial (retrocarotid) space along the clinoid line connecting the anterior and posterior processes, as in our two cases [
The premammillary artery, which is a perforating branch of posterior communicating artery (PcomA), also supplies the anterior medial thalamus. This artery normally branches from the anterior half of PcomA, which is close to the internal carotid artery, and runs outward. Therefore, there is little risk of injury unless the drain is inserted quite laterally toward the dorsal side of the internal carotid artery.
The infarctions in our two cases did not cause severe prognostic symptoms because they involved the unilateral medial thalamus. Nevertheless, the paramedian thalamic artery has a varied branching pattern, and originates from the unilateral P1 segment and terminates in the bilateral medial thalamus in 50% of cases.[
CONCLUSION
To avoid the potential for thalamic injury, neurosurgeons should consider the potential for P1 perforator injury related to cisternal drain placement. Thus, it is important to confirm the position of the basilar bifurcation preoperatively, and to not insert the drain too deeply.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Burruss JW, Hurley RA, Taber KH, Rauch RA, Norton RE, Hayman LA. Functional neuroanatomy of the frontal lobe circuits. Radiology. 2000. 214: 227-30
2. Castaigne P, Lhermitte F, Buge A, Escourolle R, Hauw JJ, Lyon-Caen O. Paramedian thalamic and midbrain infarct: Clinical and neuropathological study. Ann Neurol. 1981. 10: 127-48
3. Deeb ZL, Jannetta PJ, Rosenbaum AE, Kerber CW, Drayer BP. Tortuous vertebrobasilar arteries causing cranial nerve syndromes: Screening by computed tomography. J Comput Assist Tomogr. 1979. 3: 774-8
4. Dirk MH, Massimiliano S, Peter B, Karen W, Johannes M, Peter A. Evolution of neurological, neuropsychological and sleep-wake disturbances after paramedian thalamic stroke. Stroke. 2008. 39: 62-8
5. Flamm ES, Adams HP, Beck DW, Pinto RS, Marler JR, Walker MD. Dose-escalation study of intravenous nicardipine in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 1988. 68: 393-400
6. Fuwa I, Mayberg M, Gadjusek C, Harada T, Luo Z. Enhanced secretion of endothelin by endothelial cells in response to hemoglobin. Neurol Med Chir (Tokyo). 1993. 33: 739-43
7. Gilsbach JM, Reulen HJ, Ljunggren B, Brandt L, Holst HV, Mokry M. Early aneurysm surgery and preventive therapy with intravenously administered nimodipine: A multicenter, double-blind, dose-comparison study. Neurosurgery. 1990. 26: 458-64
8. Greitz T, Lofstedt S. The relationship between the third ventricle and the basilar artery. Acta Radiol. 1954. 42: 85-100
9. Horiuchi T, Yamamoto Y, Kuroiwa M, Rahmah NN, Hongo K. Pontine infarction caused by medial branch injury of the basilar artery as a rare complication of cisternal drain placement. J Clin Neurosci. 2012. 19: 592-3
10. Klimo P, Kestle JR, MacDonald JD, Schmidt RH. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 2004. 100: 215-24
11. Kodama N, Sasaki T, Kawakami M, Sato M, Asari J. Cisternal irrigation therapy with urokinase and ascorbic acid for prevention of vasospasm after aneurysmal subarachnoid hemorrhage. Outcome in 217 patients. Surg Neurol. 2000. 53: 110-8
12. Masanori I, Anthony DM, Katarina Z, Bruce IT, Paul LP, George CW. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006. 99: 1252-60
13. Pedroza A, Dujovny M, Ausman JI, Diaz FG, Artero JC, Berman SK. Microvascular anatomy of the interpeduncular fossa. J Neurosurg. 1986. 64: 484-93
14. Sasaki T, Kassell NF, Zuccarello M, Nakagomi T, Fijiwara S, Colohan AR. Barrier disruption in the major cerebral arteries during the acute stage after experimental subarachnoid hemorrhage. Neurosurgery. 1986. 19: 177-84
15. Smoker WR, Price MJ, Keyes WD, Corbett JJ, Gentry LR. High-resolution computed tomography of the basilar artery: 1. Normal size and position. AJNR Am J Neuroradiol. 1986. 7: 55-60
16. Tatsuya S, Namio K, Masahisa K, Masahiro S, Jun A, Yoshiharu S. Urokinase cisternal irrigation therapy for prevention of symptomatic vasospasm after aneurysmal subarachnoid hemorrhage: A study of urokinase concentration and the fibrinolytic system. Stroke. 2000. 31: 1256-62
17. Usui M, Saito N, Hoya K, Todo T. Vasospasm prevention with postoperative intrathecal thrombolytic therapy: A retrospective comparison of urokinase, tissue plasminogen activator, and cisternal drainage alone. Neurosurgery. 1994. 34: 235-45
18. Yu YL, Moseley IF, Pullicino P, McDonald WI. The clinical picture of ectasia of the intracerebral arteries. J Neurol Neurosurg Psychiatry. 1982. 45: 29-36