- Neurosurgery, Klinik Im Park, Seestrasse 220, 8027 Zurich, Switzerland
Correspondence Address:
Thomas Mindermann
Neurosurgery, Klinik Im Park, Seestrasse 220, 8027 Zurich, Switzerland
DOI:10.4103/2152-7806.196238
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Thomas Mindermann. Paraneoplastic symptoms caused by extracranial meningioma metastases?. 20-Dec-2016;7:106
How to cite this URL: Thomas Mindermann. Paraneoplastic symptoms caused by extracranial meningioma metastases?. 20-Dec-2016;7:106. Available from: http://surgicalneurologyint.com/surgicalint_articles/paraneoplastic-symptoms-caused-by-extracranial-meningioma-metastases/
Abstract
Background:There are only few reports on distant metastases of cranial meningiomas WHO I. In one-third of the cases, distant metastases seem to be clinically silent. This is the first case of distant metastases which may have manifested with a paraneoplastic syndrome.
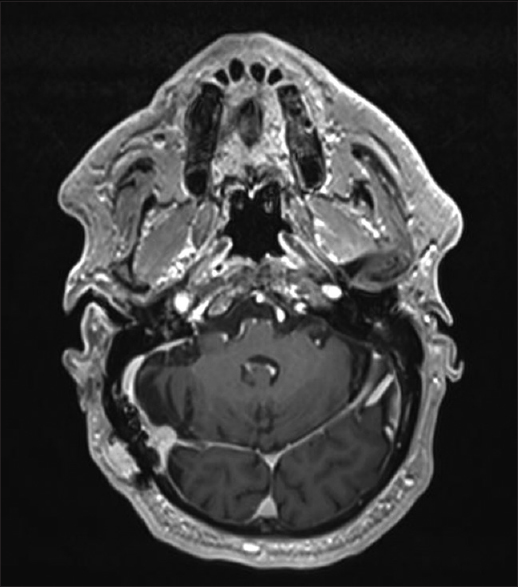
Case Description:A 52-year-old white male patient was diagnosed with distant metastases to the bones and liver 11 and 12 years following craniotomy and removal of a tentorial meningioma WHO I. At that time, the patient had developed paresthesia, unsteady gait, and a slight cognitive impairment, which in retrospect had no other explanation than that of a paraneoplastic syndrome. Eighteen years following craniotomy, a small intracranial tumor rest is under control following two single session radiosurgery treatments. At present, the patient has a multitude of bone and liver metastases, which seem to cause his paraneoplastic symptoms.
Conclusion:Screening for malignancies in patients with paraneoplastic symptoms and a history of cranial meningioma should include screening for distant metastases from the meningioma.
Keywords: Extracranial, meningioma, metastasis, paraneoplastic
INTRODUCTION
The literature on cranial meningiomas WHO I with distant metastases is rare, with many publications including meningiomas WHO II and III.[
CASE REPORT
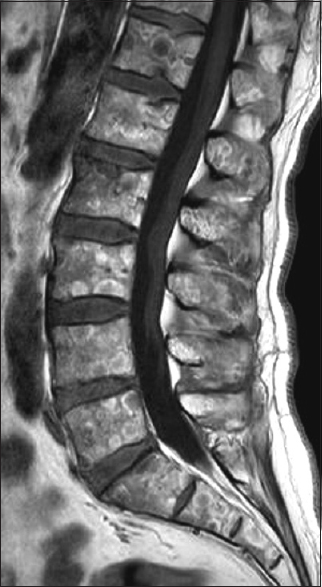
At 52 years of age, a white male patient underwent craniotomy for a meningioma WHO I of the right tentorium. The postoperative course was uneventful. At the age of 61 years and 9 years following craniotomy, the patient developed paresthesias of the four extremities, an unsteady gait, and problems in concentrating. Based on these symptoms and a neurological work up, the patient was diagnosed with peripheral small fiber polyneuropathy. At age 63 and 11 years following craniotomy, a magnetic resonance imaging (MRI) of the pelvis showed a multitude of small skeletal lesions suggestive of metastases [
DISCUSSION
The patient developed distant metastases from cranial meningioma in two of the most frequently affected locations, i.e., the bones and liver. Bone metastases may affect long bones, pelvis, skull, or vertebrae. At least pelvis and vertebrae were affected bones in the present case. The pathophysiology leading to distant metastases may be a hematogenous spread originating from tumor invasion into large venous sinuses or from surgical seeding.[
The symptoms of paresthesias, unsteady gait, and problems with concentration began approximately 9 years following craniotomy. One may speculate that those symptoms were paraneoplastic and that the cranial meningioma had metastasized already at that time. A number of paraneoplastic symptoms have been attributed to cranial meningiomas such as optic neuropathy, dermatomyositis, Cushing's syndrome, nephrotic syndrome, or hypocalcemia.[
Establishing an accurate diagnosis of metastatic meningioma may be challenging considering that one-third of metastasizing meningiomas are clinically silent and that the disease may manifest with paraneoplastic symptoms, as may have been the case in this patient. At present, there are no recommendations for the treatment of distant metastases other than symptomatic interventions, e.g., in the case of pathological fractures.
CONCLUSION
This case illustrates that benign intracranial meningiomas WHO I may lead to extracranial metastases and how numerous and yet oligosymptomatic such metastases may be. Patients with distant metastases from cranial meningiomas may present with or without symptoms. They may present with local symptoms and signs caused by the lesions within affected organs or with systemic symptoms caused by paraneoplastic symptoms. To the best of my knowledge, this is the first case of paraneoplastic symptoms, which may be caused by distant metastases from an intracranial meningioma WHO I. Therefore, screening for malignancies in patients with paraneoplastic symptoms and a history of cranial meningioma should include screening for distant metastases from the meningioma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abboud M, Haddad G, Kattar M, Aburiziq I, Geara FB. Extraneural metastases from cranial meningioma: A case report. Rad Oncol. 2009. 4: 20-
2. Almodovar R, Lindo DP, Martin H, Mazzuchelli R, Pardo J, Quiros FJ. Dermatomyositis and meningioma in the same patient. Reumatol Clin. 2012. 8: 87-9
3. Baek BJ, Shin JM, Lee CK, Lee JH, Lee KH. Atypical primary meningioma in the nasal septum with malignant transformation and distant metastasis. BMC Cancer. 2012. 12: 275-
4. Basunaid S, Franssen FME, Accord R, Hamid MA, Mahesh S, Baumert BG. Case report: Pulmonary metastases of malignant meningioma. 2014. 2013. 2: 222-
5. Celenk F, Erkilic S, Durucu C, Baysal E, Kanlikama M. Late metastasis of an intracranial meningioma to the hard palate. J Craniofac Surg. 2012. 23: 1912-4
6. Delgado-Lopez PD, Martin-Velasco V, Castilla-Diez JM, Fernandez-Arconada O, Corrales-Garcia EM, Galacho-Harnero A. Metastatic meningioma to the eleventh dorsal vertebral body: Total en bloc spondylectomy. Case report and review of the literature. Neurochirurgia. 2006. 17: 240-9
7. Enam SA, Abdulrauf S, Mehta B, Malik GM, Mahmood A. Metastasis in meningioma. Acta Neurochir. 1996. 138: 1172-7
8. Figueroa BE, Quint DJ, McKeever PE, Chandler WF. Extracranial metastatic meningioma. Br J Radiol. 1999. 72: 513-6
9. Giraldi P, Terreni MR, Andreotti C, Losa M, Lanzi R, Pontiroli AE. Meningioma presenting with Cushing's syndrome: An unusual clinical presentation. Ann Neurol. 2003. 53: 138-4
10. Karasick JL, Mullan SF. A survey of metastatic meningiomas. J Neurosurg. 1974. 40: 206-12
11. Leemans J, van Calenbergh F, Sciot R, Debiec-Rychter M, Decaluwe H, Nackaerts K. Pulmonary metastasis of a meningioma presenting as a solitary pulmonary nodule: 2 case reports. Acta Clin Belg. 2016. 6: 1-4
12. Mazzei TS, Mazzei CM, Sanoner A. Hypocalcemia and tetany in meningioma, its disappearance after removal of the tumor. Paraneoplastic syndrome. Rev Clin Esp. 1976. 141: 183-6
13. Nakano S, Kanamori A, Nakamura M, Mizukawa K, Negi A. Paraneoplastic neuropathy associated with cerebellar choroid meningioma. Eye. 2013. 27: 1220-1
14. Surov A, Gottschling S, Bolz J, Kornhuber M, Alfieri A, Holzhausen HJ. Distant metastases in meningioma: An underestimated problem. J Neurooncol. 2013. 112: 323-7
15. Wang KD, Su YB, Zhang Y. Recurrent intracranial meningioma with multiple pulmonary metastases: A case report. Oncol Lett. 2015. 10: 2765-8
16. Wasserkrug R, Peyser E, Lichtig C. Extracranial bone metastases from intracranial meningiomas. Surg Neurol. 1979. 12: 480-4
17. Williamson BE, Stanton CA, Levine EA. Chest wall metastasis from recurrent meningioma. Am Surg. 2001. 67: 966-8
18. Zachariah PP, Mathew A, Rajesh R, Kurien G, Unni VN. Nephrotic syndrome associated with meningioma. Indian J Nephrol. 2013. 23: 63-6