- Department of Neurosurgery, Atsuchi Neurosurgical Hospital, Kagoshima, Japan,
- Department of Neurosurgery, Kagoshima University, Kagoshima, Japan,
- Department of Science, Kagoshima University, Kagoshima, Japan,
- Department of Neurologic Surgery, Mayo Clinic, Rochester, United States.
Correspondence Address:
Kazunori Arita, Department of Neurosurgery, Kagoshima University, Kagoshima, Japan.
DOI:10.25259/SNI_474_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takashi Kawahara1, Kazunori Arita2, Shingo Fujio2, Nayuta Higa2, Hiroki Hata3, FM Moinuddin4, Ryosuke Hanaya2. Patients of idiopathic normal-pressure hydrocephalus have small dural sac in cervical and upper thoracic levels: A supposed causal association. 10-Nov-2023;14:391
How to cite this URL: Takashi Kawahara1, Kazunori Arita2, Shingo Fujio2, Nayuta Higa2, Hiroki Hata3, FM Moinuddin4, Ryosuke Hanaya2. Patients of idiopathic normal-pressure hydrocephalus have small dural sac in cervical and upper thoracic levels: A supposed causal association. 10-Nov-2023;14:391. Available from: https://surgicalneurologyint.com/surgicalint-articles/12635/
Abstract
Background: Idiopathic normal pressure hydrocephalus (iNPH) is a neurological disorder presenting a triad including dementia and ventricular enlargement. The mechanism causing excessive cerebrospinal fluid (CSF) accumulation in the ventricles in iNPH is poorly understood. We hypothesized that the age-related degradation of the spinal shock-absorbing system composed of a spinal dural sac (SDS) and surrounding soft tissue, preventing ventricular enlargement caused by wide CSF pulsation driven by heartbeats, may be involved in the ventricular enlargement observed in iNPH.
Methods: Sixty-four patients with iNPH in their seventies who underwent a lumboperitoneal shunt and a control group of 79 people in the same age group who underwent brain check-ups were included in the study. We compared the sizes of the cervical and upper parts of the thoracic SDS using magnetic resonance imaging between the two groups.
Results: The anterior-posterior distances of the dural sac at C5 were shorter in patients with iNPH of both sexes than those in the control group (P = 0.0008 in men and P = 0.0047 in women). The number of disc levels with disappeared CSF space surrounding the cervical cord was more in iNPH (P = 0.0176 and P = 0.0003). The midsagittal area of the upper part of the spinal sac, C2-Th4, was smaller in iNPH (P = 0.0057 and P = 0.0290).
Conclusion: Narrowing of the cervical dural sac and midsagittal area in the upper part of the SDS in patients with iNPH may reflect the degradation of the shock-absorbing mechanism for CSF pressure pulsations, which may cause iNPH or at least aggravate iNPH by other unknown causes.
Keywords: Cervical spondylosis, Idiopathic normal pressure hydrocephalus, Shock absorber, Spinal dural sac
INTRODUCTION
Idiopathic normal pressure hydrocephalus (iNPH) is a neurological disorder characterized by nonobstructive ventricular enlargement without a precedent causative event and mainly presents with disturbances in gait, micturition control, and cognition. As it primarily affects older adults, 60–80 years old, its prevalence is increasing in aging societies.[
Through the treatment of a large number of iNPH patients, 40–60 patients a year, at our Normal Pressure Hydrocephalus Center, Atsuchi Neurosurgical Hospital, Kagoshima, Japan, we frequently encounter iNPH patients with spinal column disorders, including cervical spondylosis, leading to narrowing of the spinal dural sac (SDS). Due to the high incidence of such an accompaniment, we hypothesized that the narrowing of the SDS is not a mere coincidence associated with iNPH but a causative or aggravating factor. In addition, our previous study on spontaneous intracranial hypotension (SIH) showed that the SDS shrinks even in the early stages of the disease, suggesting that the SDS acts as a shock absorber for intracranial CSF pressure change.[
MATERIALS AND METHODS
At our normal pressure hydrocephalus center, Atsuchi Neurosurgical Hospital, 96 patients underwent lumbarperitoneal shunt surgery for iNPH between January 2021 and July 2022. Among them, 64 consecutive patients (36 men and 28 women) in their seventies, the largest age group, were selected for this study. For the controls, 79 participants (50 men and 29 women) in the same age group were selected from the 661 people who underwent BCU in our hospital during the same period. In the patient group, in addition to brain scans, cervical-level magnetic resonance (MR) images and upper thoracic-level MR images were routinely obtained to rule out cryptic causes of hydrocephalus. In the control group, in addition to whole brain scans, T2-weighted midsagittal cervical MR images were routinely obtained in all 79 examinees, while T2-weighted midsagittal MR images at the upper thoracic level were obtained in 55.
As a surrogate for the total volume of the SDS, we evaluated the above mentioned size of the upper part of the SDS, cervical, and upper thoracic vertebral levels in both groups.
Neuroradiologic features, including the Evans index, distances from the vertebral body to the spinous process at the mid-C5 level, number of disc levels with vanished CSF space surrounding the cervical cord, and midsagittal cervical and upper thoracic intradural area (C2 bottom-Th4 bottom), were compared between the two groups for each sex [
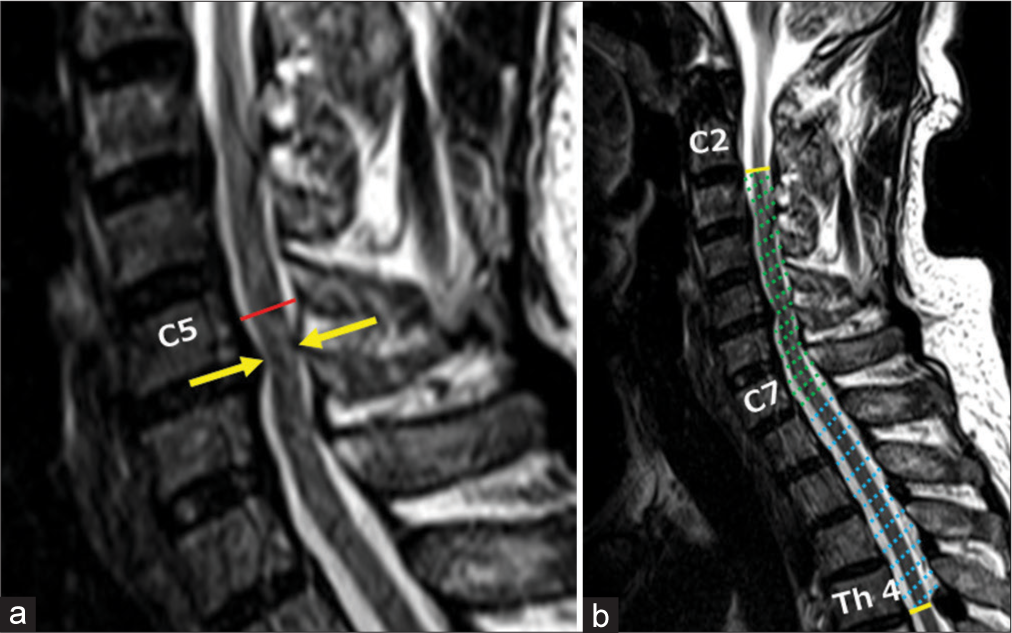
Figure 1:
Measurements of magnetic resonance imaging features at cervical and upper thoracic levels. (a) Red line: Distance (mm) from the mid-C5 vertebral body to the mid-C5 spinous process. Yellow arrows: Number of disc levels at which the CSF space surrounding the cervical spinal cord vanishes, one in this case. (b) Dotted area: Midsagittal area (mm2) from C2 bottom to Th4 bottom.
Statistical analyses
Statflex® version 6.0 software program (Artech Japan) was used for statistical analysis of the results. Data were analyzed using the Mann–Whitney U-test. Differences were considered statistically significant at P < 0.05.
Ethical consideration
This noninterventional study was approved by the hospital’s medical ethics committee (R4-2, October 2022). This study was conducted according to the principles of the Declaration of Helsinki, as revised in 2000, and the ethical guidelines for medical and health research involving human subjects (effective February 9, 2015) promulgated by the Ministry of Health, Welfare and Labor, Japan. The data were analyzed in a de-identified manner to protect patient privacy.
RESULTS
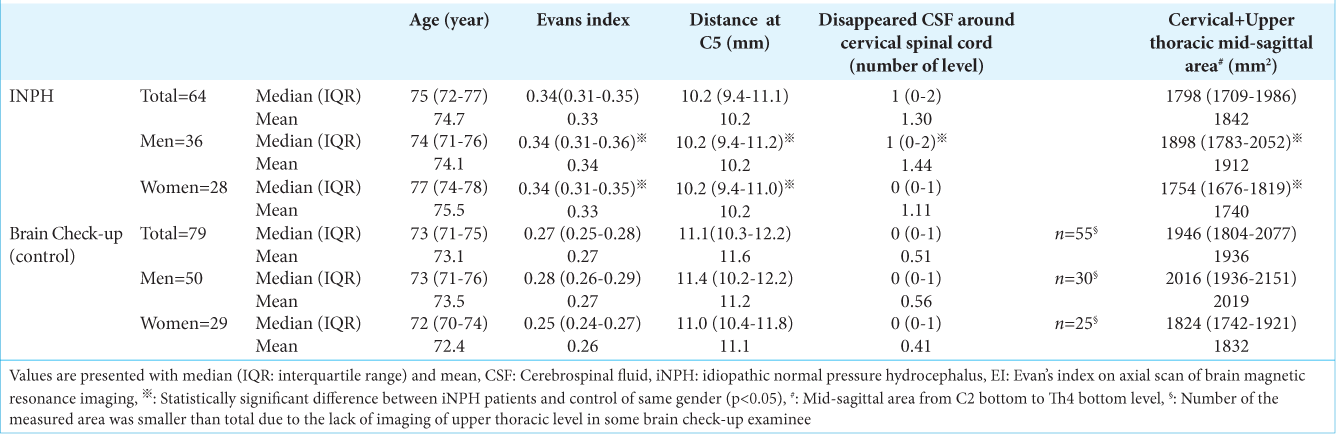
The Evans index was significantly higher in the iNPH group than that in the BCU group in both sexes: men (median 0.34 vs. 0.28, P < 0.001) and women (median 0.34 vs. 0.25, P < 0.001) [
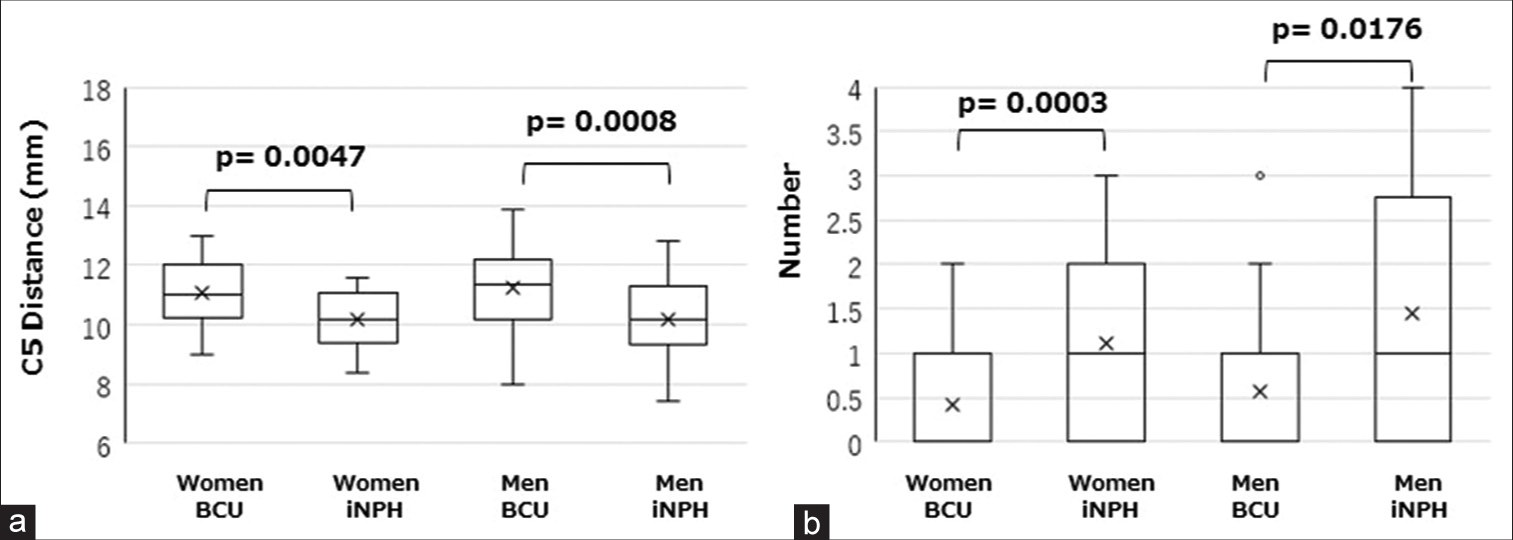
The distances from the midvertebral body to the spinous process at the C5 level were significantly shorter in the iNPH group than those in the BCU group in both sexes: men (median 10.2 vs. 11.4 mm, P = 0.0008) and women (median 10.2 vs. 11.0 mm, P = 0.0047) [
Figure 2:
Comparison of cervical magnetic resonance imaging findings between patients with idiopathic normal-pressure hydrocephalus (iNPH) and controls (brain check-up (BCU), examinees of brain check-up). (a) The distance from the mid-C5 vertebral body to the mid-C5 spinous process in patients with iNPH was significantly shorter than in controls of both sexes. (b) The number of disc levels with vanished cerebrospinal fluid space surrounding the cervical spinal cord was significantly higher in patients with iNPH than in the controls of both sexes. The values are presented by box plot.
The number of disc levels with vanished CSF space surrounding the cervical cord was significantly more in the iNPH group than in the BCU group in both sexes: men (mean 1.44 vs. 0.56; median one vs. 0, P = 0.0176) and women (mean 1.11 vs. 0.41; median one vs. 0, P = 0.0003) [
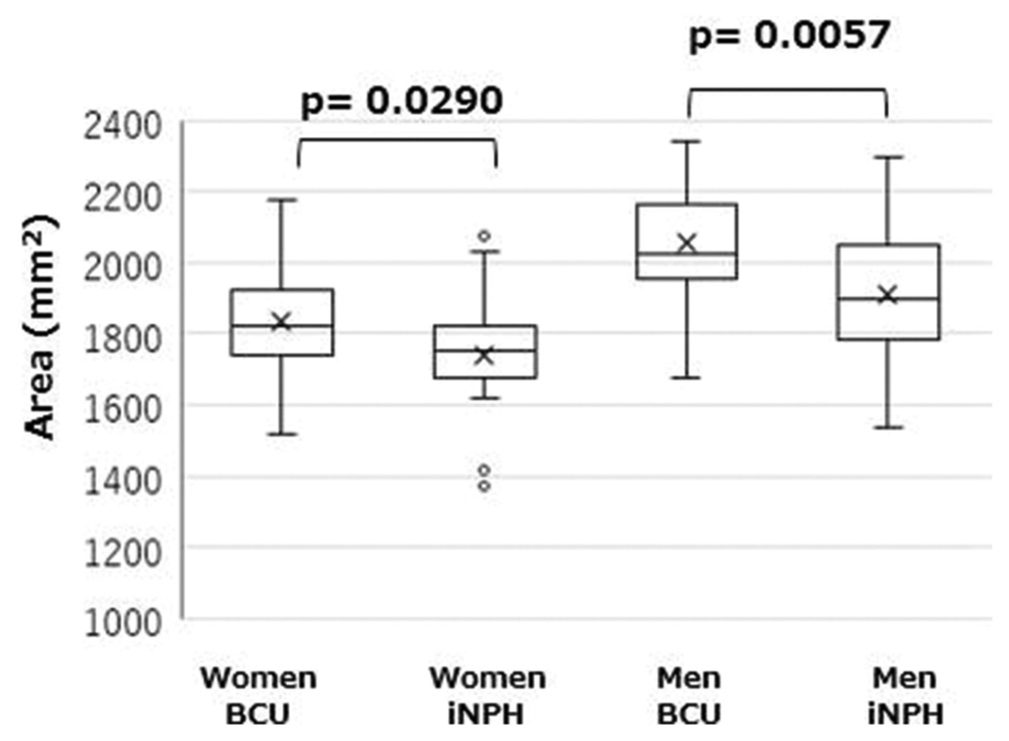
The midsagittal cervical to the upper thoracic intradural area (C2 bottom-Th4 bottom) was significantly smaller in the iNPH group than that in the BCU group in both sexes; men (median 1898 vs. 2016 mm2, P = 0.0057) and women (median 1754 vs. 1824 mm2, P = 0.0290) [
DISCUSSION
In this study, as only the sagittal MR images of the upper part of the spine that are included in routine imaging for iNPH and BCU were available, we measured the size of the upper part of the SDS as a surrogate of the whole volume of the SDS. We found a significantly narrow subarachnoid space surrounding the cervical spinal cord and a smaller SDS in patients with iNPH compared to examinees of the BCU. These results are consistent with our previous observation of the high prevalence of a narrow spinal canal in patients with iNPH.[
The mechanism of hydrocephalus development without a particular cause remains unclear. Some researchers have argued that age-related decay in the CSF absorption mechanism involving arachnoid granules may play a role in developing iNPH.[
Before discussing the mechanism underlying the development of iNPH, we consider the following facts:
First, the brain expands and shrinks due to changes in the intracerebral blood volume according to the cardiac beat. These brain volume changes cause wide CSF pulsations, providing wide-pressure pulsations on the brain surface.[
Second, an animal model of a unilateral large craniotomy of the normal brain (decompressed brain) showed enlargement of the ventricle on the decompressed side, suggesting that a large movement of the brain results in enlargement of the ventricles.[
Recent research has found that large CSF pulsation pressure on the ventricular wall causes enlargement of the ventricles in cases of obstructed CSF flow, noncommunicating hydrocephalus.[
Third, the CSF volume in the SDS is larger than that in the cranium, at approximately 75 mL versus 25 mL.[
Fourth, the spinal epidural space between the two layers of the dura mater contains soft tissue, including fat and venous plexus without valves, functioning like a cushion. Therefore, the SDS may act as a shock absorber that lessens the intracranial CSF pressure pulsations driven by the cardiac beats. Our previous study also suggested the existence of this mechanism; the SDS shrinks before changes in intracranial structures due to low CSF pressure in patients with SIH. In contrast, the two layers of the dura mater in the cranium are almost inseparable and firmly attached to the skull; thus, they do not act as shock absorbers.[
Therefore, if the cervical subarachnoid space becomes narrow enough to prevent the CSF from moving freely or the whole SDS smaller, the spinal shock-absorbing mechanism cannot cancel the large intracranial CSF pressure pulsations. This degradation of the shock-absorbing mechanism may enhance CSF pressure pulsations on the inner and outer brain surfaces, eventually resulting in the ventricular and subarachnoid space enlargement typically seen in iNPH patients. This speculation seems supported by the fact that iNPH occurs primarily in older adults in their 60s to 80s, in whom degenerative spondylosis is common.[
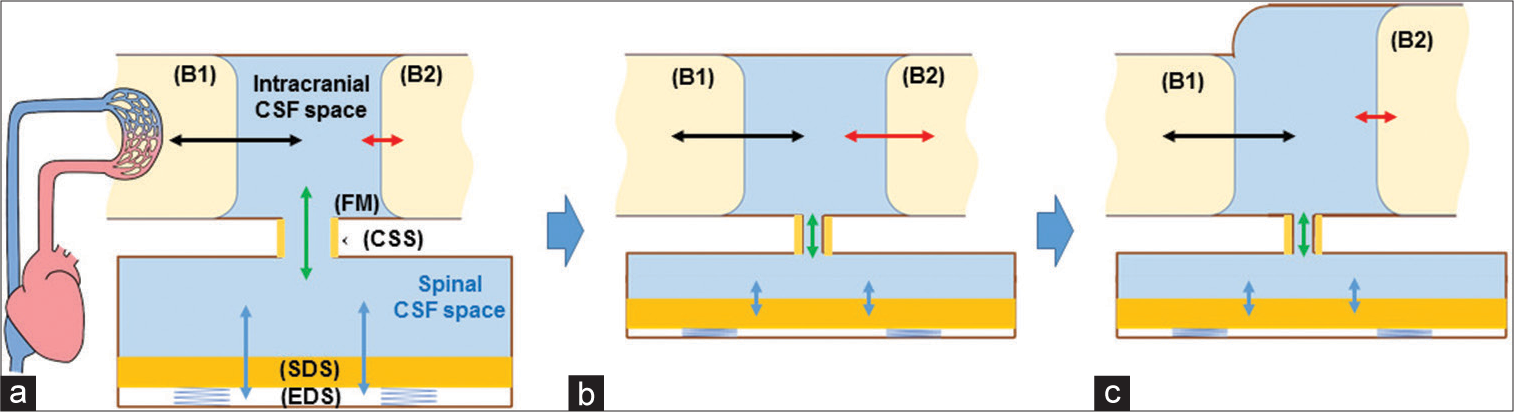
The development mechanism of iNPH uses a dual-chamber model, intracranial CSF space, and spinal CSF space [
Figure 4:
Conceptual illustration of causative relationship between narrow spinal dural sac (SDS) and idiopathic normal pressure hydrocephalus (iNPH). (a) The Physiological pressure-absorbing mechanism that prevents the brain surface (B2) from receiving large cerebrospinal fluid (CSF) pressure pulsations (black arrow) driven by intracerebral blood volume changes (B1). The pulsations are largely canceled (red arrow) by large spinal dural sac (SDS) pulsations (blue arrows) surrounded by the epidural space (EDS) including soft tissue, owing to a quick CSF shift (green arrow) through the foramen magnum (FM) and cervical subarachnoid space (CSS). B1: Brain model generating CSF pressure pulses; B2: Brain model receiving CSF pressure pulses. (b) When the cervical part of the dural sac or the total dural sac becomes smaller due to vertebral spondylosis, the cancelation mechanism by CSF shift (green arrow) through foramen magnum will be diminished (blue arrows), and the brain surface (B2) will directly receive large CSF pressure pulsations (red arrow). (c) Large CSF pressure pulsations cause enlargement of the inner and outer brain surfaces (B2), leading to iNPH. The enlargement of the brain surface itself lessens the CSF pulsations (red arrow), leading to a steady width of the brain surface. Black arrow: CSF pressure driven by intracerebral blood volume changes, Green arrow: diminished CSF shift through foramen magnum.
When spinal degenerative changes occur, the narrowing of the cervical subarachnoid space causes a decrease in flow velocity. It limits the movement of the CSF into and out of the SDS (green arrow in [
As a result, the increased CSF pressure pulsations increase the ventricle sizes and some parts of the cisterns [
Limitations
There were many inherent limitations in this retrospective survey.
First, we did not measure the volume of the entire SDS or the cervical subarachnoid space volume.
Second, an association between iNPH and various patient clinicopathological features, such as cerebral white matter disease, small vessel disease, arteriosclerosis, hypertension, hypercholesterolemia, diabetes, and excessive alcohol consumption, has been reported.[
Third, the exact mechanism by which wide CSF pressure pulsations cause enlargement of the brain surface and impair brain function is not well understood.
Therefore, longitudinal prospective follow-up of patients with vertebral spondylosis using modern imaging techniques and elaborate cognitive function tests may help to understand the mechanism of iNPH development.
CONCLUSION
Our retrospective comparison between patients with iNPH and examinee of BCU, both in their seventies, revealed a narrow CSF cervical subarachnoid space and upper SDS in patients with iNPH. These changes in patients with iNPH may indicate degradation of the shock absorber, composed of the SDS and surrounding epidural tissue, compensating for the wide intracranial pressure pulsations. The shock absorber decay may play an important role in developing iNPH. Future prospective studies on patients with spondylosis must validate our findings.
Ethical approval
The author(s) declare that they have taken the ethical approval from IEC.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Commentary
This is an interesting clinical study where the investigators performed upper spine MRI studies on iNPH patients and an age-matched “control” cohort. They hypothesized that iNPH had a more narrowed cervical-upper thoracic spinal thecal sac compared to non-iNPH subjects. By multiple measures, they found statistically significant differences supporting the hypothesis.
The authors acknowledge that a methodological limitation is that the analysis was limited to midline sagittal T2 imaging rather than volumetric CSF axial studies. I agree. This is just one of many unanswered questions. Is the spinal stenosis acquired (degenerative) or congenital, or both? Why was the analysis limited to the upper spine? Should not the same hypothesis pertain to the entire spine?
The authors also acknowledge that the etiology of iNPH is unknown and almost certainly multifactorial. Stated otherwise, it is likely a “multihit” phenomenon. Their data rather convincingly suggest that spinal stenosis may be one of the “hits.” However, they do not acknowledge that there are millions of patients with cervical spinal stenosis, much worse than demonstrated here, who do not have iNPH.
In the discussion, the authors present a series of “facts.” I would argue that none of them are “facts” but instead speculations and observations. For example, the statement that the brain expands and contracts with changes in CBV is misleading. Most of the arterial vasculature runs in the subarachnoid and cisternal spaces. These spaces likely contribute to the expansion/ contraction much more than the brain parenchyma itself, where only tiny perforators run. Therefore, the next statement as to the source of CSF pulsations is in question as well.
Marvin Bergsneider, MD
Professor, Director of the UCLA Pituitary and Skullbase Tumor Program
University of California at Los Angeles
300 Stein Plaza, Suite 420
Los Angeles, CA 90095
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Andersson J, Rosell M, Kockum K, Lilja-Lund O, Söderström L, Laurell K, editors. Prevalence of idiopathic normal pressure hydrocephalus: A prospective, population-based study. PLoS One. 2019. 14: e0217705
2. Cushing H. Studies on the cerebro-spinal fluid: I. Introduction. J Med Res. 1914. 31: 1-19
3. Edsbagge M, Starck G, Zetterberg H, Ziegelitz D, Wikkelso C. Spinal cerebrospinal fluid volume in healthy elderly individuals. Clin Anat. 2011. 24: 733-40
4. Engel DC, Pirpamer L, Hofer E, Schmidt R, Brendle C. Incidental findings of typical iNPH imaging signs in asymptomatic subjects with subclinical cognitive decline. Fluids Barriers CNS. 2021. 18: 37
5. Hiraoka K, Meguro K, Mori E. Prevalence of idiopathic normal-pressure hydrocephalus in the elderly population of a Japanese rural community. Neurol Med Chir (Tokyo). 2008. 48: 197-99
6. Israelsson H, Larsson J, Eklund A, Malm J. Risk factors, comorbidities, quality of life, and complications after surgery in idiopathic normal pressure hydrocephalus: Review of the INPH-CRasH study. Neurosurg Focus. 2020. 49: E8
7. Kawahara T, Arita K, Fujio S, Hanaya R, Atsuchi M, Moinuddin FM. Dural sac shrinkage signs on magnetic resonance imaging at the thoracic level in spontaneous intracranial hypotension-its clinical significance. Acta Neurochir (Wien). 2021. 163: 2685-94
8. Kawahara T, Atsuchi M, Arita K, Fujio S, Higa N, Moinuddin FM. Dural sac shrinkage signs on spinal magnetic resonance imaging indicate overdrainage after lumboperitoneal shunt for idiopathic normal pressure hydrocephalus. Surg Neurol Int. 2022. 13: 269
9. Kawahara T, Atsuchi M, Arita K, Fujio S, Higa N, Hanaya R. Paravertebral cerebrospinal fluid exudation in young women with postdural puncture headache: A hypothetical interpretation based on anatomical study on intervertebral foramen. Asian J Neurosurg. 2023. 18: 117-24
10. Last RJ, Tompsett DH. Casts of the cerebral ventricles. Br J Surg. 1953. 40: 525-43
11. Leinonen V, Vanninen R, Rauramaa T. Cerebrospinal fluid circulation and hydrocephalus. Handb Clin Neurol. 2017. 145: 39-50
12. Leinonen V, Kuulasmaa T, Hiltunen M. iNPH-the mystery resolving. EMBO Mol Med. 2021. 13: e13720
13. Matsumae M, Kikinis R, Mórocz I, Lorenzo AV, Albert MS, Black PM. Intracranial compartment volumes in patients with enlarged ventricles assessed by magnetic resonance-based image processing. J Neurosurg. 1996. 84: 972-81
14. Matsumoto T, Nagai H, Fukushima T, Mase M. Analysis of intracranial pressure pulse wave in experimental hydrocephalus. Childs Nerv Syst. 1994. 10: 91-5
15. Nakajima M, Kuriyama N, Miyajima M, Ogino I, Akiba C, Kawamura K. Background risk factors associated with shunt intervention for possible idiopathic normal pressure hydrocephalus: A nationwide hospital-based survey in Japan. J Alzheimers Dis. 2019. 68: 735-44
16. Nakajima M, Yamada S, Miyajima M, Ishii K, Kuriyama N, Kazui H, editors. Guidelines for management of idiopathic normal pressure hydrocephalus (Third Edition): Endorsed by the Japanese society of normal pressure hydrocephalus. Neurol Med Chir (Tokyo). 2021. 61: 63-97
17. Oresković D, Klarica M, Vukić M. The formation and circulation of cerebrospinal fluid inside the cat brain ventricles: A fact or an illusion?. Neurosci Lett. 2002. 327: 103-6
18. Preuss M, Hoffmann KT, Reiss-Zimmermann M, Hirsch W, Merkenschlager A, Meixensberger J. Updated physiology and pathophysiology of CSF circulation--the pulsatile vector theory. Childs Nerv Syst. 2013. 29: 1811-25
19. Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011. 128: 309-16
20. Stoquart-ElSankari S, Balédent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007. 27: 1563-72
21. Telano LN, Baker S, editors. Physiology, cerebral spinal fluid. StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. p.
22. Tsunoda A, Mitsuoka H, Sato K, Kanayama S. A quantitative index of intracranial cerebrospinal fluid distribution in normal pressure hydrocephalus using an MRI-based processing technique. Neuroradiology. 2000. 42: 424-9
23. Tucker A, Kajimoto Y, Ohmura T, Ikeda N, Furuse M, Nonoguchi N. Fluoroscopic-guided paramedian approach for lumbar catheter placement in cerebrospinal fluid shunting: Assessment of safety and accuracy. Oper Neurosurg (Hagerstown). 2019. 16: 471-7
24. Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009. 117: 1-14
25. Yamada S, Tsuchiya K, Bradley WG, Law M, Winkler ML, Borzage MT. Current and emerging MR imaging techniques for the diagnosis and management of CSF flow disorders: A review of phase-contrast and time-spatial labeling inversion pulse. AJNR Am J Neuroradiol. 2015. 36: 623-30
26. Yamada S, Mase M. Cerebrospinal fluid production and absorption and ventricular enlargement mechanisms in hydrocephalus. Neurol Med Chir (Tokyo). 2023. 63: 141-51