- Department of Surgery, Division of Neurosurgery, Cardiothoracic Surgical Unit, Lagos State, Nigeria.
- Department of Paediatrics, Cardiothoracic Surgical Unit, Lagos State, Nigeria.
- Department of Anesthesiology, Cardiothoracic Surgical Unit, Lagos State, Nigeria.
- Department of Surgery, Cardiothoracic Surgical Unit, Lagos State, Nigeria.
- Department of Surgery, Division of Otorhinolaryngology, College of Medicine, University of Lagos, Lagos State, Nigeria.
- Department of Surgery, Neurosurgery Unit, Lagos University Teaching Hospital, Lagos State, Nigeria.
- Department of Microbiology, College of Medicine, University of Lagos, Idi-Araba, Lagos State, Nigeria.
Correspondence Address:
Okezie Obasi Kanu, Department of Surgery, Division of Neurosurgery, College of Medicine, University of Lagos, Lagos, Nigeria.
DOI:10.25259/SNI_605_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Okezie Obasi Kanu1, Omotayo Ojo1, Christopher Esezobor2, Olufemi Bankole1, John Olatosi3, Ezekiel Ogunleye4, Chinyere Asoegwu5, Morgan Eghosa6, Bamidele Adebayo6, Rita Oladele7, Clement Nwawolo5. Pediatric brain abscess – etiology, management challenges and outcome in Lagos Nigeria. 08-Dec-2021;12:592
How to cite this URL: Okezie Obasi Kanu1, Omotayo Ojo1, Christopher Esezobor2, Olufemi Bankole1, John Olatosi3, Ezekiel Ogunleye4, Chinyere Asoegwu5, Morgan Eghosa6, Bamidele Adebayo6, Rita Oladele7, Clement Nwawolo5. Pediatric brain abscess – etiology, management challenges and outcome in Lagos Nigeria. 08-Dec-2021;12:592. Available from: https://surgicalneurologyint.com/surgicalint-articles/11281/
Abstract
Background: Brain abscess in children is a neurosurgical emergency with potentially catastrophic outcome despite the advances made in neuroimaging techniques and antibiotic therapy. Symptoms are nonspecific and may vary with the child’s age, location, size, numbers and stage of abscess, and the primary source of infection. Treatment is usually with broad-spectrum antibiotics in combination and surgical evacuation in most cases or antibiotics alone in selected cases with clear-cut indications. This study was to document clinical characteristics, etiological factors, and spectrum of bacteriologic agents responsible for pediatric brain abscess in an African city, the challenges and management outcome over the study period.
Methods: This was a retrospective study over an 11-year period involving 89 children who presented with brain abscess. Information of interest was extracted from the medical records of each participant. The results from data analysis were presented in charts and tables.
Results: Eighty-nine children aged 0.85–15.7 years (median age of 6.4 years) met the inclusion criteria. The male-to-female ratio was 1.8:1. Headache (80%), fever (78%), and hemiparesis (78%) were the most common symptoms. Brain imaging deployed was CT scan in 56 (63%), MRI in 9 (10%), and transfontanel ultrasound scan in 24 (27%) children. Seventy-one (80%) children had antibiotics with surgical evacuation while 18 (20%) children received only antibiotics. In 19 (27%) children, the culture of the abscess was negative. In 53 (75%) children, Gram-positive aerobic organisms were isolated. A total of 75 patients (84%) had a favorable outcome.
Conclusion: Pediatric brain abscess still poses significant public health challenge, especially in resource-limited regions. Successful management of brain abscess requires high index of suspicion for early diagnosis, referral, and intervention.
Keywords: Africa, Antibiotic, Brain abscess, Children, Gram-positive aerobes
INTRODUCTION
Despite the significant decline in mortality rate with advancements in diagnostic imaging and neurosurgical techniques, brain abscesses remain a potentially fatal central nervous system infection. Published literature reveals that approximately 25% of all brain abscesses are seen in childhood, mostly in the age group of 4–7 years.[
Brain abscess may be asymptomatic at the early stage. Clinical findings may be unspecific, mild or severe, and may be influenced by the patient’s age, the stage, size and location of abscess, presence of meningitis, and patient’s immune status. [
The main microorganisms responsible for brain abscess are aerobic and anaerobic streptococci and staphylococci. Others are Bacteroides species, Proteus species, Haemophilus influenzae, Escherichia coli,Citrobacter group, Nocardia,Aspergillus, and Corynebacterium species and Mycobacterium tuberculosis.[
A combination of broad-spectrum or organism-specific antimicrobial therapy and surgical drainage is the preferred therapeutic method in most cases. Antimicrobial therapy alone is used for small-sized lesions usually <2.5 cm, multiple abscesses, and deep-seated lesions.[
The objective of this study was to determine the clinical characteristics, predisposing factors, and management challenges and outcome of brain abscess in children treated in an urban African city over an 11-year period.
MATERIALS AND METHODS
This retrospective study included children with cerebral abscess treated in three facilities between January 2006 and December 2016. Lagos state is the most densely populated state in Nigeria with multiethnic and multinational representations. It is the economic capital of Nigeria with a population of over 18 million. Ethical clearance was obtained from the relevant authority (Reference No ADM/ DCST/ HREC/APP/1977).
Brain abscess was defined as a single or multifocal lesion located in the cerebrum, cerebellum, or midbrain, identifiable on computerized tomography (CT), magnetic resonance imaging (MRI) scan, or transfontanel ultrasound scan (TFUSS) which met at least one of the following criteria:
Neuroradiological findings suggesting brain abscess with clinical response to antimicrobial therapy Purulent material within the defined lesion at surgery A positive culture of intracerebral material Microscopic or histologic features of an abscess.
The age, gender, clinical features at presentation, the locations and the causes of the abscesses where identified, and laboratory findings were recorded. Patients were either treated with antibiotics alone if the sizes were <2.5 cm or deep seated or antibiotics combined with surgical drainage if abscesses were large and readily accessible. In those with multiple abscesses, the large collections were drained and antibiotics continued. Surgical drainage was by craniotomy, burr hole, or percutaneous transfontanel aspiration. All aspirates were sent for Gram staining, culture, and sensitivity. Percutaneous aspiration was followed by CT scan or TFUSS and if the abscess increased in size postaspiration, it was repeated.
The results of microscopy, culture, and sensitivity of aspirates and abscess materials were documented. The treatment modalities, including surgical techniques, type and duration of antibiotic therapy, complications, and outcomes, were documented. The Glasgow Outcome Scale was used to determine favorable and unfavorable outcome.
RESULTS
Demographics
Eighty-nine children were included in this study, age 0.85–15.7 years (median age of 6.4 years). The average admission rate was nine cases/year. There were 57 males and 32 females with a male-to-female ratio of 1.8:1. Sixty-eight children (76%) were younger than 10 years of age.
Clinical features
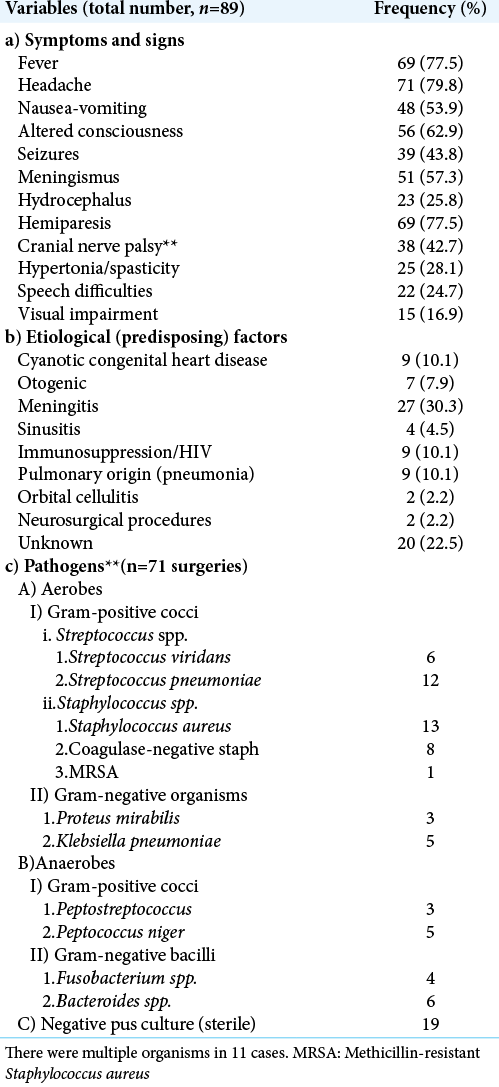
The duration of symptoms ranged from 19 to 92 days with a mean of 37.6 days. [
Etiological/predisposing factors
The underlying diseases or predisposing factors are listed on [
Diagnostic imaging and abscess characteristics
Radiologic diagnosis was made using CT scan in 56 (63%) children, (MRI) in 9 (10%), and TFUSS in 24 (27%) children. There were solitary abscesses in 63 (71%) and multiple abscesses in 26 children (29%). The abscesses were located in the supratentorial region in 69 children (78%), infratentorial in 7 children (8%), and in both regions in 9 children (10%). Of the total of 78 abscesses observed in the supratentorial region, 39 (50%) were on the left, 27 (35%) on the right, and 12 were bilateral. Some multiple abscesses affected only one hemisphere. Of the 63 cases of solitary abscesses, 62 were supratentorial, only one was infratentorial in location. Information on the specific lobe involved in each hemisphere was lacking. The multiple abscesses in the infratentorial region (15) affected the left side (6) more than the right (4) and were bilateral in five cases. The number of multiple abscesses ranged from 2 to 11.
Microbiology
Seventy-one (80%) children had surgical evacuation of the abscesses, of which 15 (21%) had negative cultures [
Treatment
Drug therapy
All patients had parenteral antibiotics for a minimum duration of 4 weeks. Total duration of antibiotic therapy was 6–8 weeks. Unless otherwise dictated by sensitivity pattern, the most common antibiotics used were cefotaxime (or ceftriaxone), amikacin (or gentamycin), and metronidazole based on presumed etiologic agent. Vancomycin was used in the patients with MRSA or as indicated by sensitivity pattern. Other antibiotics used were meropenem, cefuroxime, cefpodoxime, and cotrimoxazole. Treatment was tailored in a multidisciplinary mode, to treat both the abscess and the source in each case. Additional procedures if required were performed by the comanaging team.
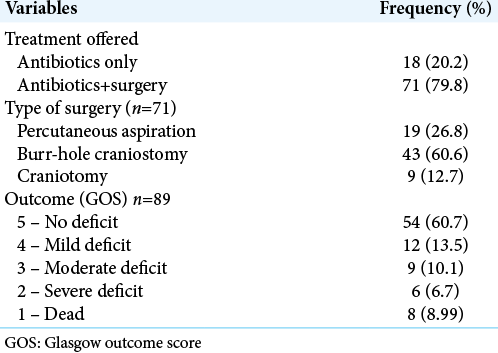
Eighteen patients (20%) received antibiotics alone while 71 patients (60%) had antibiotics and surgical drainage [
Procedures
Of the 71 patients who had surgery, percutaneous aspiration was used in 19 patients (27%), burr-hole craniostomy in 43 (61%), and craniotomy in 9 children (13%) [
Capsule excision is not routinely done at craniotomy, but three of the nine cases treated by craniotomy had partial capsule excision because they were located close to the surface.
In all cases, abscess cavity was irrigated with saline containing antibiotics. Gentamycin is our antibiotic of choice for irrigation but some cases were irrigated with vancomycin and additionally metronidazole. We did not record any case with ventricular rupture, so ventriculostomy was not needed in any of the patients in this series.
Outcome
A total of 75 patients (84%) had a favorable outcome: 54 (61%) had full recovery, 12 (14%) had mild deficits, and 9 (10.1%) had moderate deficits. Of the 14 (16%) who had unfavorable outcome, 8 (9%) died during admission while 6 (7%) had severe deficits [
DISCUSSION
With the introduction of modern imaging, antibiotics, and stereotactic surgical techniques, the outcome of brain abscess has dramatically improved.[
This study of 89 children with an average of 8.1 cases/year has higher incidence than the series published from Turkey, Korea, and the USA with a range of 1.67–2.7 cases/year.[
Clinical findings in children are mostly nonspecific, especially in the younger age group.[
There was no established predisposing factor in 20 (23%) of 89 children in this series. In published studies, this ranges from 8.3% to 40%.[
Imaging diagnosis was done with CT scan, TFUSS, and MRI in the ratio of 6.2:1:2.7. The use of TFUSS has proven very useful in our region and has been used to diagnose various intracranial conditions.[
TFUSS is unable to accurately characterize the abscess or the count in multiple lesions and this contributed to paucity of the information regarding the number of abscesses in children who underwent this procedure. Because it is operator dependent, some of the information might have been missed out. However, TFUSS will characterize large solitary lesions (>2.5 cm in diameter) and lesions closer to the surface facilitating early diagnosis and therapy. TFUSS can be repeated severally without risk of exposure to irradiation.[
Our high rate of sterile culture is not uncommon. In several series, the culture-negative rate ranges from 10% to 56%.[
The most common micro-organisms in published literature, responsible for brain abscess are aerobic and anaerobic streptococci and staphylococci.[
In this series, 20% of patients received antimicrobial therapy alone. This falls within the range of 10–24% in published literature.[
Corticosteroid use in the management of brain abscesses has attracted controversies. Some authors have opined that steroid use is associated with increased morbidity and mortality.[
Sixty-two (87%) of the 71 patients who had surgical intervention received minimally invasive procedures, while only nine patients had craniotomy. Percutaneous aspiration was performed in 19 patients while burr-hole craniostomy was used in 43 patients. Stereotactic techniques are not yet available in our center. The use of craniotomy for the treatment of abscess has significantly decreased while minimally invasive or image-guided aspiration techniques are now preferred as first surgical choice for abscesses.[
Outcome in this study was unfavorable in 16% with a mortality rate of 9% [
CONCLUSION
Brain abscess is common in children with high incidence of morbidity and mortality often related to late presentation and therefore still poses a public health challenge in resource-limited regions. Successful management of childhood brain abscess with acceptable outcome is dependent on early diagnosis and intervention, the rational use of antimicrobial therapy, and adaptation of available technology. Despite the fact that Lagos has a multiethnic and multinational population, authors acknowledge that medical and demographic variations exist between countries.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to Late James T. Goodrich MD, PhD (RIP) for his mentoring and contributions toward this study.
References
1. Aebi C, Kaufmann F, Schaad UB. Brain abscess in childhood-long-term experiences. Eur J Pediatr. 1991. 150: 282-6
2. Atiq M, Ahmed US, Allana SS, Chishti KN. Brain abscess in children. Indian J Pediatr. 2006. 73: 401-4
3. Auvichayapat N, Auvichayapat P, Aungwarawong S. Brain abscess in infants and children: A retrospective study of 107 patients in northeast Thailand. J Med Assoc Thai. 2007. 90: 1601-7
4. Boviatsis EJ, Kouyialis AT, Stranjalis G, Korfias S, Sakas DE. CT-guided stereotactic aspiration of brain abscesses. Neurosurg Rev. 2003. 26: 206-9
5. Canpolat M, Ceylan O, Per H, Koc G, Tumturk A, Kumandas S. Brain abscesses in children: Results of 24 children from a reference center in Central Anatolia, Turkey. J Child Neurol. 2015. 30: 458-67
6. Cochrane DD. Consultation with the specialist. Brain abscess. Pediatr Rev. 1999. 20: 209-15
7. Cole TS, Clark ME, Jenkins AJ, Clark JE. Pediatric focal intracranial suppuration: A UK single-center experience. Childs Nerv Syst. 2012. 28: 2109-14
8. Felsenstein S, Williams B, Shingadia D, Coxon L, Riordan A, Demetriades AK. Clinical and microbiologic features guiding treatment recommendations for brain abscesses in children. Pediatr Infect Dis J. 2013. 32: 129-35
9. Finkelstein JA, Christiansen CL, Platt R. Fever in pediatric primary care: Occurrence, management, and outcomes. Pediatrics. 2000. 105: 260-6
10. Frazier JL, Ahn ES, Jallo GI. Management of brain abscesses in children. Neurosurg Focus. 2008. 24: E8
11. Garvey G. Current concepts of bacterial infections of the central nervous system. Bacterial meningitis and bacterial brain abscess. J Neurosurg. 1983. 59: 735-44
12. Gelabert-Gonzalez M, Serramito-Garcia R, Garcia-Allut A, Cutrin-Prieto J. Management of brain abscess in children. J Paediatr Child Health. 2008. 44: 731-5
13. Goodkin HP, Harper MB, Pomeroy SL. Intracerebral abscess in children: Historical trends at Children’s Hospital Boston. Pediatrics. 2004. 113: 1765-70
14. Hakan T, Ceran N, Erdem I, Berkman MZ, Goktas P. Bacterial brain abscesses: An evaluation of 96 cases. J Infect. 2006. 52: 359-66
15. Kagawa M, Takeshita M, Yato S, Kitamura K. Brain abscess in congenital cyanotic heart disease. J Neurosurg. 1983. 58: 913-7
16. Kanu OO, Esezobor CI, Ojo OA, Asoegwu CN, Nnoli C, Dawang Y. Infantile supratentorial subdural empyema managed by percutaneous aspiration: An outcome study in a Nigerian city. Sudan J Paediatr. 2019. 19: 37-43
17. Kanu OO, Nnoli C, Olowoyeye O, Ojo O, Esezobor C, Adeyomoye A. Infantile subdural empyema: The role of brain sonography and percutaneous subdural tapping in a resource-challenged region. J Neurosci Rural Pract. 2014. 5: 355-9
18. Kao KL, Wu KG, Chen CJ, Wu JJ, Tang RB, Chang KP. Brain abscesses in children: Analysis of 20 cases presenting at a medical center. J Microbiol Immunol Infect. 2008. 41: 403-7
19. Kutlay M, Colak A, Yildiz S, Demircan N, Akin ON. Stereotactic aspiration and antibiotic treatment combined with hyperbaric oxygen therapy in the management of bacterial brain abscesses. Neurosurgery. 2005. 57: 1140-6
20. Kutlay M, Colak A, Yildiz S, Demircan N, Akin ON. Stereotactic aspiration and antibiotic treatment combined with hyperbaric oxygen therapy in the management of bacterial brain abscesses. Neurosurgery. 2008. 62: 540-6
21. Lee CG, Kang SH, Kim YJ, Shin HJ, Choi HS, Lee JH. Brain abscess in Korean children: A 15-year single center study. Korean J Pediatr. 2010. 53: 648-52
22. Mamelak AN, Mampalam TJ, Obana WG, Rosenblum ML. Improved management of multiple brain abscesses: A combined surgical and medical approach. Neurosurgery. 1995. 36: 76-85
23. Mampalam TJ, Rosenblum ML. Trends in the management of bacterial brain abscesses: A review of 102 cases over 17 years. Neurosurgery. 1988. 23: 451-8
24. Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997. 25: 763-79
25. Miller ES, Dias PS, Uttley D. CT scanning in the management of intracranial abscess: A review of 100 cases. Br J Neurosurg. 1988. 2: 439-46
26. Miniar T, Amel BA, Khalil S, Ben Helal BH, Gueddiche GM, Tilouche TS. Pyogenic brain abscess in children: A Tunisian multi-center experience. Afr Health Sci. 2018. 18: 560-8
27. Nathoo N, Nadvi SS, Narotam PK, van Dellen JR. Brain abscess: Management and outcome analysis of a computed tomography era experience with 973 patients. World Neurosurg. 2011. 75: 716-26
28. Ozsurekci Y, Kara A, Cengiz AB, Celik M, Ozkaya-Parlakay A, Karadag-Oncel E. Brain abscess in childhood: A 28-year experience. Turk J Pediatr. 2012. 54: 144-9
29. Pandian JD, Moosa NV, Cherian PJ, Radhakrishnan K. Brainstem abscess complicating tetralogy of Fallot successfully treated with antibiotics alone. Neurol India. 2000. 48: 272-5
30. Pit S, Jamal F, Cheah FK. Microbiology of cerebral abscess: A four-year study in Malaysia. J Trop Med Hyg. 1993. 96: 191-6
31. Puthucheary SD, Parasakthi N. The bacteriology of brain abscess: A local experience in Malaysia. Trans R Soc Trop Med Hyg. 1990. 84: 589-92
32. Ratnaike TE, Das S, Gregson BA, Mendelow AD. A review of brain abscess surgical treatment-78 years: Aspiration versus excision. World Neurosurg. 2011. 76: 431-6
33. Saez-Llorens X. Brain abscess in children. Semin Pediatr Infect Dis. 2003. 14: 108-14
34. Sarmast AH, Showkat HI, Bhat AR, Kirmani AR, Kachroo MY, Mir SF. Analysis and management of brain abscess; a ten year hospital based study. Turk Neurosurg. 2012. 22: 682-9
35. Seydoux C, Francioli P. Bacterial brain abscesses: Factors influencing mortality and sequelae. Clin Infect Dis. 1992. 15: 394-401
36. Shachor-Meyouhas Y, Bar-Joseph G, Guilburd JN, Lorber A, Hadash A, Kassis I. Brain abscess in children-epidemiology, predisposing factors and management in the modern medicine era. Acta Paediatr. 2010. 99: 1163-7
37. Sheehan JP, Jane JA, Ray DK, Goodkin HP. Brain abscess in children. Neurosurg Focus. 2008. 24: E6
38. Simjian T, Muskens IS, Lamba N, Yunusa I, Wong K, Veronneau R. Dexamethasone administration and mortality in patients with brain abscess: A systematic review and meta-analysis. World Neurosurg. 2018. 115: 257-63
39. Takeshita M, Kagawa M, Yonetani H, Izawa M, Yato S, Nakanishi T. Risk factors for brain abscess in patients with congenital cyanotic heart disease. Neurol Med Chir (Tokyo). 1992. 32: 667-70
40. Tseng JH, Tseng MY. Brain abscess in 142 patients: Factors influencing outcome and mortality. Surg Neurol. 2006. 65: 557-62
41. Tsou TP, Lee PI, Lu CY, Chang LY, Huang LM, Chen JM. Microbiology and epidemiology of brain abscess and subdural empyema in a medical center: A 10-year experience. J Microbiol Immunol Infect. 2009. 42: 405-12
42. Wu S, Wei Y, Yu X, Peng Y, He P, Xu H. Retrospective analysis of brain abscess in 183 patients: A 10-year survey. Medicine (Baltimore). 2019. 98: e17670
43. Xiao F, Tseng MY, Teng LJ, Tseng HM, Tsai JC. Brain abscess: Clinical experience and analysis of prognostic factors. Surg Neurol. 2005. 63: 442-9
44. Yang SY. Brain abscess: A review of 400 cases. J Neurosurg. 1981. 55: 794-9
45. Yogev R, Bar-Meir M. Management of brain abscesses in children. Pediatr Infect Dis J. 2004. 23: 157-9
46. Zhang C, Hu L, Wu X, Hu G, Ding X, Lu Y. A retrospective study on the aetiology, management, and outcome of brain abscess in an 11-year, single-centre study from China. BMC Infect Dis. 2014. 14: 311