- Department of Neurosurgery, University Hospital of Fez, Morocco.
Correspondence Address:
Marouane Hammoud, Assistant Professor, Department of Neurosurgery, University hospital of Fez, Morocco.
DOI:10.25259/SNI_809_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Marouane Hammoud, Oualid Mohammed Hmamouche, Faycal Lakhdar, Mohammed Benzagmout, Khalid Chakour, Mohammed El Faiz Chaoui. Pediatric extra-axial glioblastoma with bone invasion leading to a subcutaneous mass: A case report. 26-Jan-2024;15:25
How to cite this URL: Marouane Hammoud, Oualid Mohammed Hmamouche, Faycal Lakhdar, Mohammed Benzagmout, Khalid Chakour, Mohammed El Faiz Chaoui. Pediatric extra-axial glioblastoma with bone invasion leading to a subcutaneous mass: A case report. 26-Jan-2024;15:25. Available from: https://surgicalneurologyint.com/surgicalint-articles/12723/
Abstract
Background: Pediatric glioblastoma multiforme (p-GBM) is an exceptionally rare and aggressive brain tumor, with even fewer reported cases with radiographic and intraoperative characteristics that mimic those of extra-axial lesions, often posing a diagnostic challenge. Despite advancements in imaging technologies, the diagnosis of GBM can still be intricate, relying primarily on histopathological confirmation.
Case Description: We present a unique case of a 15-year-old female who presented to our hospital with a new-onset focal-to-bilateral tonic-clonic seizure described as clonic movements of her left hemicorps; on clinical examination, a subcutaneous mass was evident in the right parietal region. Magnetic resonance imaging of the brain revealed a sizable extra-axial enhancing mass measuring 9 cm, located in the right parieto-occipital region with notable bone invasion. Moreover, the intraoperative findings revealed an extra-axial mass attached to the dura. Total en bloc resection was achieved. The histopathological analysis confirmed the diagnosis of glioblastoma multiforme. Subsequently, the patient underwent adjuvant radiotherapy in conjunction with temozolomide chemotherapy. Postoperatively, she exhibited clinical improvement and remained stable throughout the 6-month follow-up period.
Conclusion: We present the first case of extra-axial p-GBM in a young patient, which remarkably led to the destruction of the bone and finally resulted in a sizable parietal subcutaneous lesion in the absence of prior surgery or radiation.
Keywords: Bone invasion, Extra-axial, Pediatric glioblastoma, Subcutaneous mass
INTRODUCTION
Glioblastoma multiforme (GBM) is the most prevalent and aggressive primary malignant brain tumor in adults, accounting for approximately 45% of all primary brain tumors. However, it remains a rare entity in the pediatric population, constituting only 3–15% of primary central nervous system (CNS) tumors in children.[
Typically, GBM is intra-axially located in the deep white matter of the supratentorial region, primarily within the frontal and temporal lobes.[
To the best of our knowledge, cases involving the destruction of the dura and calvaria caused by an extra-axial pediatric GBM (p-GBM) without any prior history of surgery or radiation have not been reported previously. This occurrence may raise considerations of extra-axial tumors, such as aggressive meningiomas, and highlights the exceptional nature of this case.
CASE DESCRIPTION
A 15-year-old female high school student presented with a 1-month history of persistent holocranial headaches and intermittent vomiting. She experienced a new-onset focal-to-bilateral tonic-clonic seizure characterized by clonic movements of her left hemicorps that secondarily progressed to a convulsion. On clinical examination, all findings were normal, except for the presence of a palpable subcutaneous mass in the right parietal region.
Initial laboratory tests yielded unremarkable results.
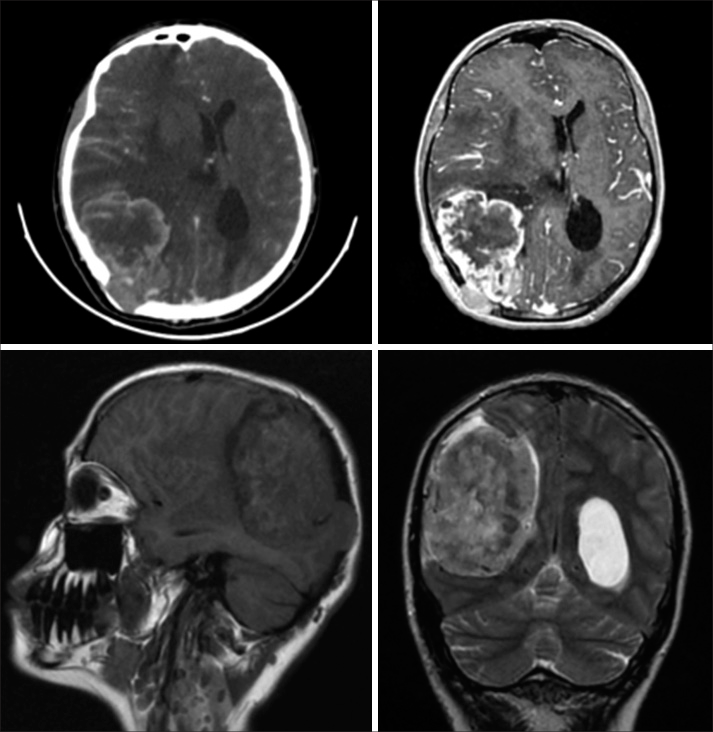
Brain computed tomography (CT) scan and magnetic resonance imaging (MRI) revealed a large, heterogeneous contrast-enhancing mass located in the right parieto-occipital region. This mass extended extra-axially, reaching up to the occipital horn medially and involving the cortical bone of the inner table laterally, displaying signs of bone invasion [
Figure 1:
Preoperative brain computed tomography scan and brain magnetic resonance imaging show a large, heterogeneous contrast-enhancing mass located in the right parieto-occipital region. This mass extended extra-axially, reaching up to the occipital horn medially and involving the cortical bone of the inner table laterally.
Based on these findings, a preoperative diagnosis of aggressive meningioma was initially considered.
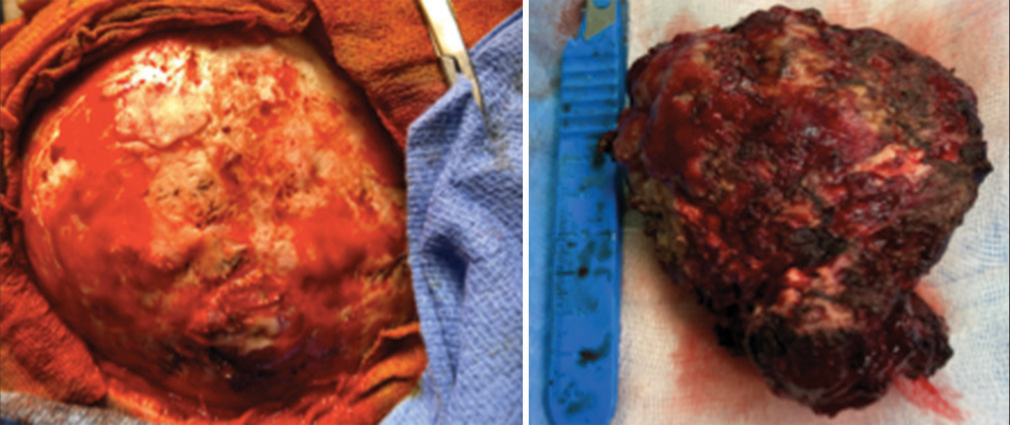
Subsequently, a Mitre’s flap and a right parieto-occipital craniotomy were performed. Intraoperatively, it was observed that the tumor had invaded and destroyed the surrounding bone [
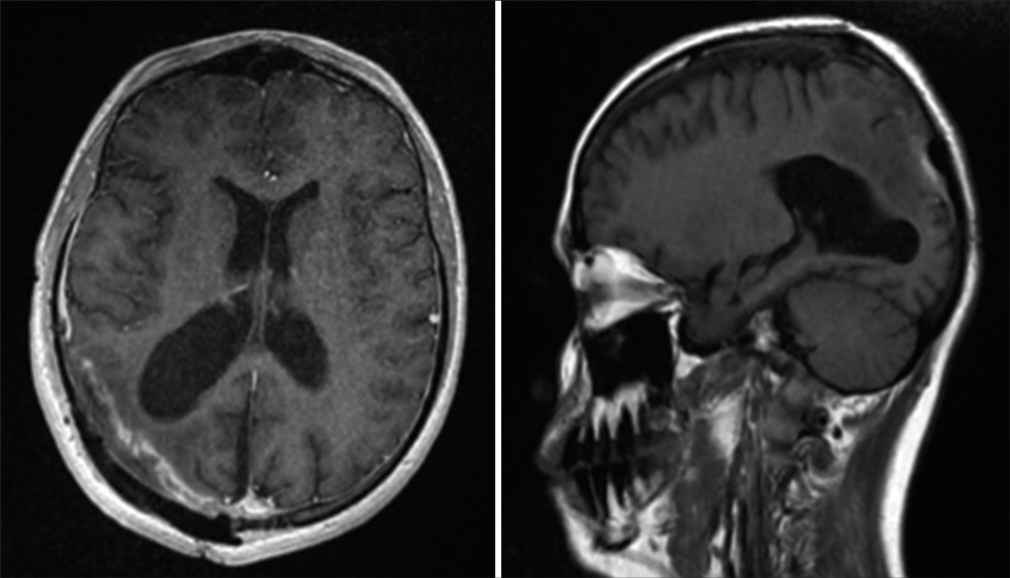
Postoperative brain MRI confirmed complete tumor resection [
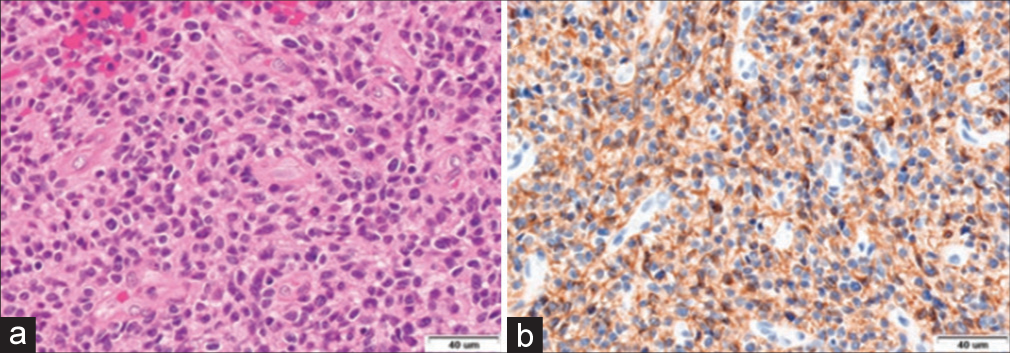
The histological analysis identified the tumor as a glioblastoma (World Health Organization – grade IV), characterized by small cells with marked angiogenesis and wide necrotic areas infiltrating both cerebral structures and meningeal sheaths [
Following the surgical procedure, the patient underwent radiotherapy and received concurrent temozolomide treatment, followed by adjuvant temozolomide therapy. In the most recent examination conducted six months post-surgery, the patient was confirmed to be progressing favorably. She remained free from seizures and had successfully returned to school.
DISCUSSION
Glioblastoma multiforme (GBM) is the most prevalent and aggressive primary brain tumor in the adult population, accounting for approximately 45% of all primary brain tumors.[
Pediatric glioblastomas (p-GBMs) are typically diagnosed during the second decade of life, with rare cases even reported during fetal development. The peak incidence of p-GBMs is observed between the ages of 15 and 19, likely reflecting the cumulative impact of various genetic factors contributing to tumorigenesis.[
From a pathophysiological standpoint, GBMs originate from astrocytes, the primary glial component of the CNS. Both macroscopic and microscopic characteristics of pediatric GBMs (p-GBMs) closely resemble those found in adults.[
GBMs are typically located in supratentorial cerebral lobes, such as the frontal and temporal lobes. Extracranial occurrences are rare; however, secondary dissemination within the brain or leptomeninges occurs in nearly 17% of patients.[
Neuroimaging plays a pivotal role in diagnosing, managing, and prognosticating GBMs. CT and MRI are fundamental tools for radiological assessment of these tumors.
However, MRI findings associated with p-GBM lack specificity. Typically, these tumors appear as heterogeneous masses with ill-defined borders, exerting a mass effect on neighboring structures and displaying varying degrees of enhancement (complex, variable, or occasionally absent). Regarding signal characteristics, p-GBM may exhibit iso- to hypointense T1 signals relative to gray matter and heterogeneously hyperintense T2 signals, often accompanied by surrounding edema, visible on fluid attenuation inversion recovery images. In cases with hemorrhage, distinct signal characteristics may emerge, including T1 hyperintensity, T2 hypointensity, low signal on T2*, and susceptibility-weighted imaging features.[
On the other hand, primary extra-axial involvement of glioblastoma (GBM) is exceedingly uncommon, with only ten/nine documented cases, including the current one. Instances of GBM with bone invasion are even scarcer. Sakata et al. reported a singular case of GBM in an elderly patient, where the disease naturally progressed to invade and destroy the skull bone.[
To the best of our knowledge, this is the first reported case of pediatric GBM with bone invasion.
The bone erosion is related to a chronic mass effect.[
To date, bone invasion in primary GBM has predominantly been observed in patients who had previously undergone surgical procedures, biopsies, or radiation therapy. These iatrogenic interventions facilitated a path for the tumor to extend beyond the confines of the brain into extracerebral structures.[
Based on the updated WHO classification of brain tumors, GBM is now classified as per the IDH gene mutation status.
GBM can, therefore, be IDH wild type or IDH mutation positive. The latter group comprises de novo lesions that primarily affect elderly patients, whereas the former group represents secondary GBMs. However, research on pediatric high-grade gliomas, such as GBMs, has shown that IDH mutations are extremely rare, especially in younger children. In other words, p-GBM is virtually always IDH wild type, but, as Pollack et al. have shown, teenagers and younger adults may have a higher frequency of secondary GBM (IDH mutant).[
The current gold standard for the treatment of adult GBMs involves maximal surgical resection followed by a combination of chemoradiotherapy and/or radiotherapy.
No well-defined standard of care has been established for pediatric glioblastomas, particularly concerning surgical management.[
Based on the largest single-center experience with pediatric glioblastoma (p-GBM), achieving maximal tumor removal is a strong predictor in improving both progression-free survival and overall survival in p-GBM.[
However, the selection of adjuvant treatment remains a subject of debate in pediatric glioblastomas due to concerns about the potential adverse effects of radiation therapy on the developing brain, as well as the variability in outcomes associated with different chemotherapy regimens.[
Radiation therapy has emerged as the standard of treatment, especially for children aged three and older who are diagnosed with a new GBM. Nevertheless, younger children, due to their increased susceptibility to the adverse effects of radiation therapy, typically receive chemotherapy as a standalone treatment, often in combination with radiation-sparing approaches.[
Recent advancements in oncology treatment have introduced novel therapeutic approaches, including molecular-targeted therapy and immunotherapy, which have become valuable additions to the arsenal for managing cancer patients.[
In the context of pediatric brain tumors, despite numerous clinical trials, molecular-targeted therapy has, with a few exceptions, not yet demonstrated a substantial impact on the survival or quality of life for children with these tumors.[
Irrespective of the treatment approach chosen, “pediatric glioblastoma” continues to present as a devastating disease, with median survival durations spanning from 13 to 73 months and a 5-year survival rate of <20%.[
CONCLUSION
We report a unique case of pediatric glioblastoma (GBM) located in an extradural location, presenting with an atypical manifestation that led to the invasion and subsequent destruction of the skull bone, all without any prior surgical or radiation intervention.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Belfquih H, Slioui B, Azami MA, Akhaddar A. Low-grade astrocytoma causing dural and calvarial destruction. Asian J Neurosurg. 2023. 18: 223-7
2. Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: Two challenges for the pediatric oncologist. Oncologist. 2004. 9: 197-206
3. Das KK, Kumar R, editors. Pediatric glioblastoma. Glioblastoma. Brisbane, AU: Codon Publications; 2017. p.
4. Das KK, Mehrotra M, Nair AP, Kumar S, Srivastava A, Sahu RN. Pediatric glioblastoma: Clinico-radiological profile and factors affecting the outcome. Childs Nerv Syst. 2012. 28: 2055-62
5. Faury D, Nantel A, Dunn SE, Guiot MC, Haque T, Hauser P. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007. 25: 1196-208
6. Forsyth TM, Bi WL, Abedalthagafi M, Dunn IF, Chiocca EA. Extracranial growth of glioblastoma multiforme. J Clin Neurosci. 2015. 22: 1521-3
7. Foster JB, Madsen PJ, Hegde M, Ahmed N, Cole KA, Maris JM. Immunotherapy for pediatric brain tumors: Past and present. Neuro Oncol. 2019. 21: 1226-38
8. Gonçalves FG, Viaene AN, Vossough A. Advanced magnetic resonance imaging in pediatric glioblastomas. Front Neurol. 2021. 12: 733323
9. Hamilton JD, Rapp M, Schneiderhan T, Sabel M, Hayman A, Scherer A. Glioblastoma multiforme metastasis outside the CNS: Three case reports and possible mechanisms of escape. J Clin Oncol. 2014. 32: e80-4
10. Osborn RE, Ley CE. Astrocytoma with calvarial erosion. AJNR Am J Neuroradiol. 1986. 7: 178
11. Packer RJ, Kilburn L. Molecular-targeted therapy for childhood brain tumors: A moving target. J Child Neurol. 2020. 35: 791-8
12. Perkins SM, Rubin JB, Leonard JR, Smyth MD, El Naqa I, Michalski JM. Glioblastoma in children: A single-institution experience. Int J Radiat Oncol Biol Phys. 2011. 80: 1117-21
13. Pollack IF, Hamilton RL, Sobol RW, Nikiforova MN, Lyons-Weiler MA, LaFramboise WA. IDH1 mutations are common in malignant gliomas arising in adolescents: A report from the children’s oncology group. Childs Nerv Syst. 2011. 27: 87-94
14. Sakata S, Arai K, Kawamata T. A subcutaneous mass due to a glioblastoma which invaded and destroyed the bone: A case report. Interdiscip Neurosurg. 2021. 25: 101194
15. Sanders RP, Kocak M, Burger PC, Merchant TE, Gajjar A, Broniscer A. High-grade astrocytoma in very young children. Pediatr Blood Cancer. 2007. 49: 888-93
16. Suri V, Das P, Jain A, Sharma MC, Borkar SA, Suri A. Pediatric glioblastomas: A histopathological and molecular genetic study. Neuro Oncol. 2009. 11: 274-80
17. Zahir ST, Mortaz M, Yazdi MB, Sharahjin NS, Shabani M. Calvarium mass as the first presentation of glioblastoma multiforme: A very rare manifestation of high-grade glioma. Neurochirurgie. 2018. 64: 76-8