- Medical College, Aga Khan University, Karachi, Pakistan.
- Department of Neurological Surgery, Aga Khan University, Karachi, Pakistan.
- Department of Histopathology, Aga Khan University, Karachi, Pakistan.
Correspondence Address:
Syed Ather Enam, Department of Neurological Surgery, The Aga Khan University Hospital, Karachi, Pakistan.
DOI:10.25259/SNI_1128_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Fatima Mustansir1, Meher Angez1, Mohammad Hamza Bajwa2, Saira Fatima3, Syed Ather Enam2. Pediatric intracranial calcified arteriovenous malformation: A case report. 20-Jan-2022;13:28
How to cite this URL: Fatima Mustansir1, Meher Angez1, Mohammad Hamza Bajwa2, Saira Fatima3, Syed Ather Enam2. Pediatric intracranial calcified arteriovenous malformation: A case report. 20-Jan-2022;13:28. Available from: https://surgicalneurologyint.com/surgicalint-articles/11348/
Abstract
Background: Brain arteriovenous malformations (AVMs) are intracranial lesions that consist of a complex tangle of abnormal blood vessels. They can occasionally become hard and calcified. This may render these lesions difficult to resect and lead to neurological complications. There are very few reported cases of calcified brain AVMs in the literature.
Case Description: We report the case of an 11-year-old patient who presented with headaches and seizures exacerbated in the past 3 months. Preoperative imaging confirmed a large, right parasagittal AVM, with significant internal calcifications seen on the computed tomography angiogram. We performed a successful microsurgical resection of the calcified AVM and confirmed the diagnosis on histopathological analysis.
Conclusion: Dense internal calcifications within AVMs are a clinical rarity and can be challenging cases for microsurgical resection.
Keywords: Aneurysm, Arteriovenous malformation, Calcified arteriovenous malformation, Cerebrovascular
INTRODUCTION
Brain arteriovenous malformations (AVMs) are rare intracranial vascular lesions consisting of a nidus of vessels traversed by arteries and veins without intervening capillaries. This anatomical arrangement leads to extensive shunting of blood between the arterial and venous systems.[
Dystrophic calcifications within AVMs are a common finding on radiographs, particularly on computed tomography (CT) scans, as they have an increased sensitivity for calcifications.[
CASE PRESENTATION
An 11-year-old boy presented to the outpatient neurosurgery clinic at a tertiary care hospital with a 3-month history of seizures and severe headaches. The patient’s family described two episodes of generalized tonic-clonic seizures lasting for 2–3 minutes each. The patient had also reported experiencing intermittent headaches for the past 2 years which had gradually increased in severity over the past 3 months. These more recent headaches were dull in character, radiating to the neck, and associated with nausea and vomiting. He had a history of asthma that was controlled on medications. He was born to consanguineously married parents. The patient’s family history was otherwise unremarkable. On initial presentation, his physical examination, including a complete neurological examination, was unremarkable.
Initial magnetic resonance imaging (MRI) from an outside hospital showed a right parasagittal lesion with postcontrast enhancement that was suggestive of a highly vascular pathology. Differential diagnoses based on radiology included hemangiopericytoma and a lipomatous lesion. He was prescribed divalproex sodium, which was unsuccessful in controlling his seizures.
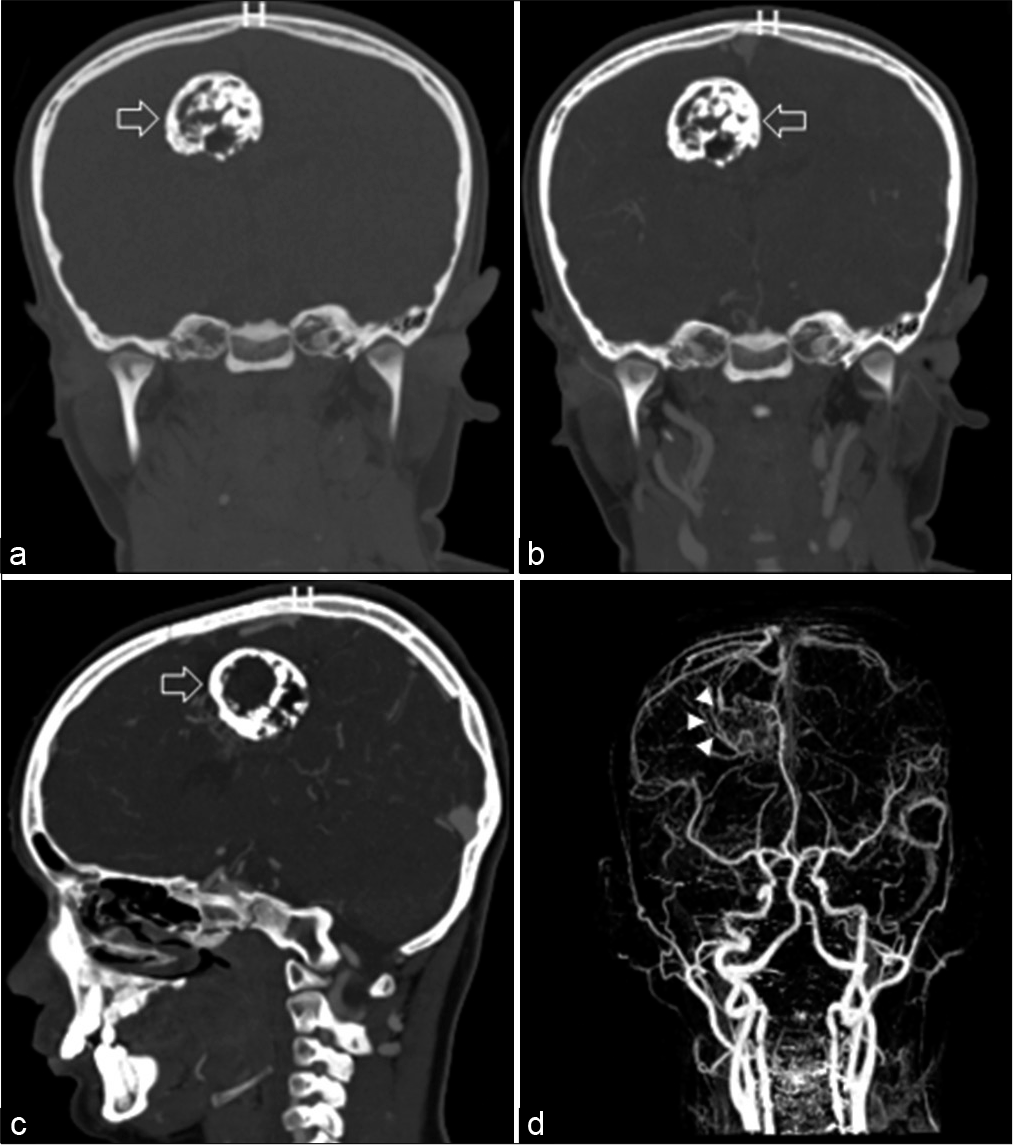
Based on these findings, the patient underwent a CT angiogram (CTA) for further investigation of a vascular etiology. CTA revealed a well-defined heterogeneous lesion in the right parasagittal region with significant internal calcification [
Figure 1:
Computed tomography head with coronal noncontrast (a), coronal postcontrast (b), and sagittal postcontrast (c) images showing a well-defined heterogeneous lesion in the right parasagittal region (white arrows) with significant internal calcifications. Flow voids are suggestive of an arteriovenous malformation (AVM) with the nidus measuring approximately 3.8 × 3.4 × 3.7 cm. CTA with coronal reconstruction (d) confirms the location of the AVM in the right frontal parasagittal region (white arrowheads).
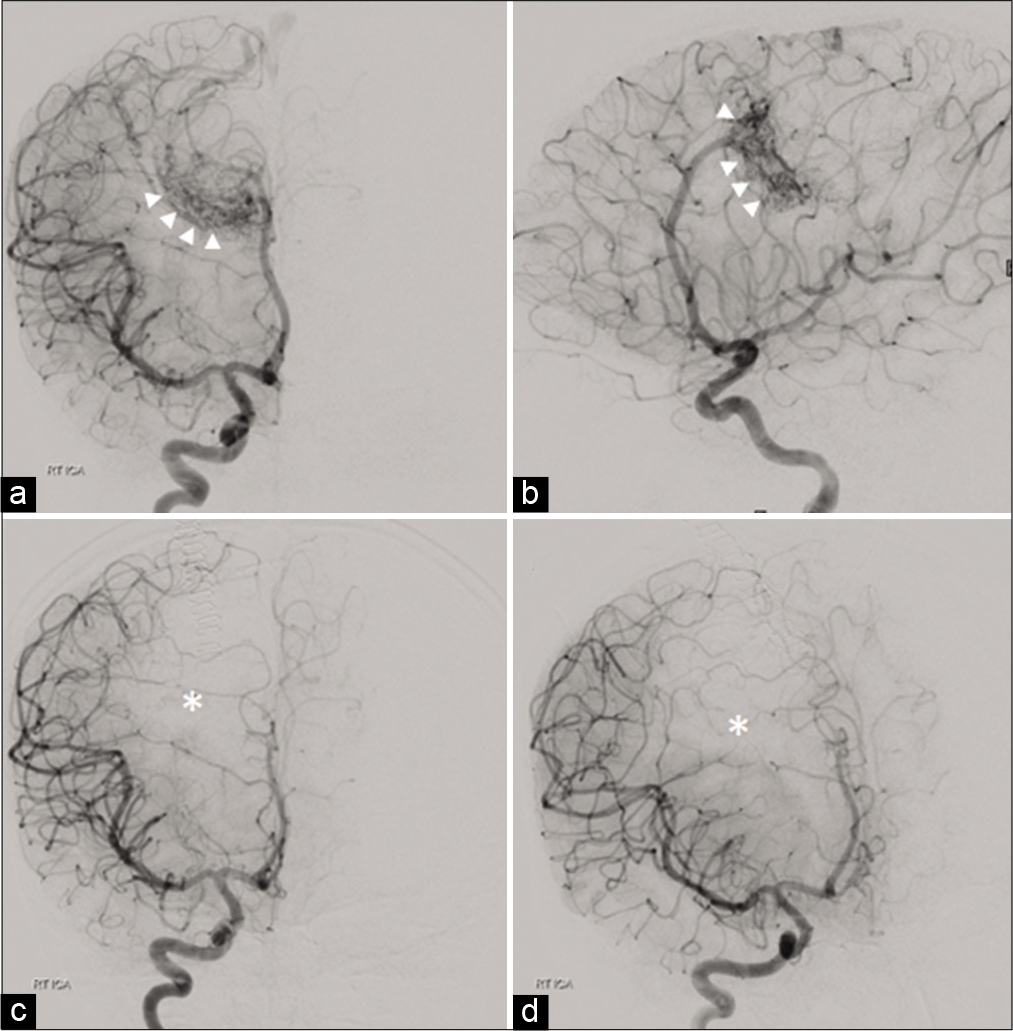
Figure 2:
Anteroposterior (a) and lateral (b) right internal carotid angiogram showing a lesion (white arrowheads) supplied by the distal branches of the right cerebral artery, mainly branches of the right pericallosal artery. The arteriovenous malformation (AVM) is shown to be draining into the superior sagittal sinus through a superficial draining vein. Postoperative angiography (c and d) shows complete resection with no arterial blush seen at the prior site of AVM (white asterisk).
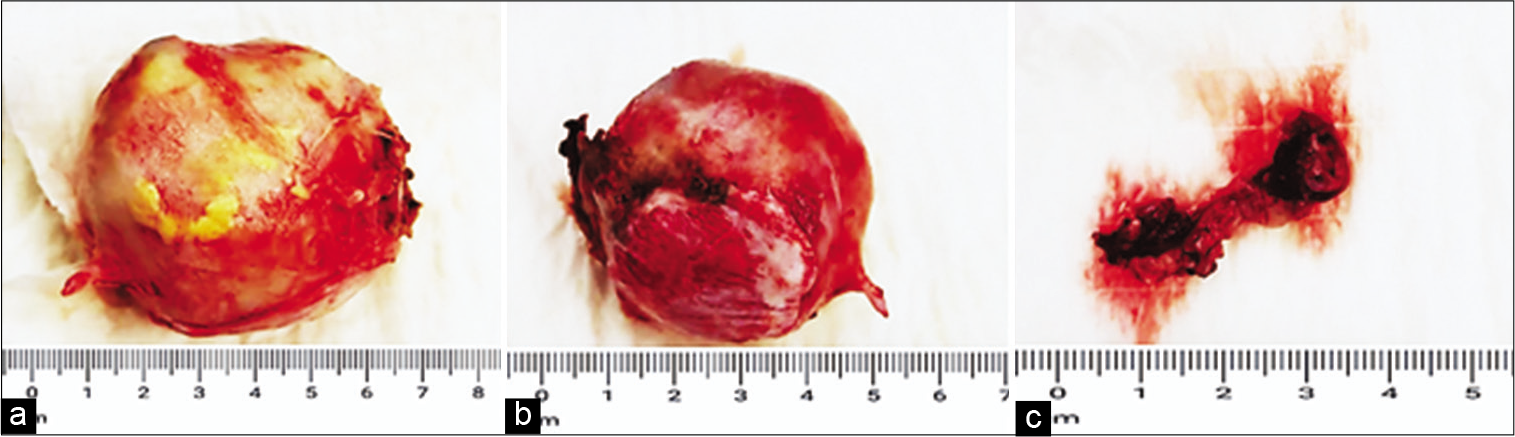
After discussing the risks and benefits of stereotactic radiosurgery and conventional surgical resection, the patient’s family opted for surgery. The patient underwent a neuronavigation-guided right frontal craniotomy under general anesthesia. Intraoperative findings included a hard, calcified, and well-encapsulated lesion which was adherent to the falx cerebri. The nidus of the AVM was confirmed to be at the anterolateral margin. The lesion was separated from the brain parenchyma along the falx cerebri and collateral vessels were coagulated. The AVM was resected en bloc along with its nidus [
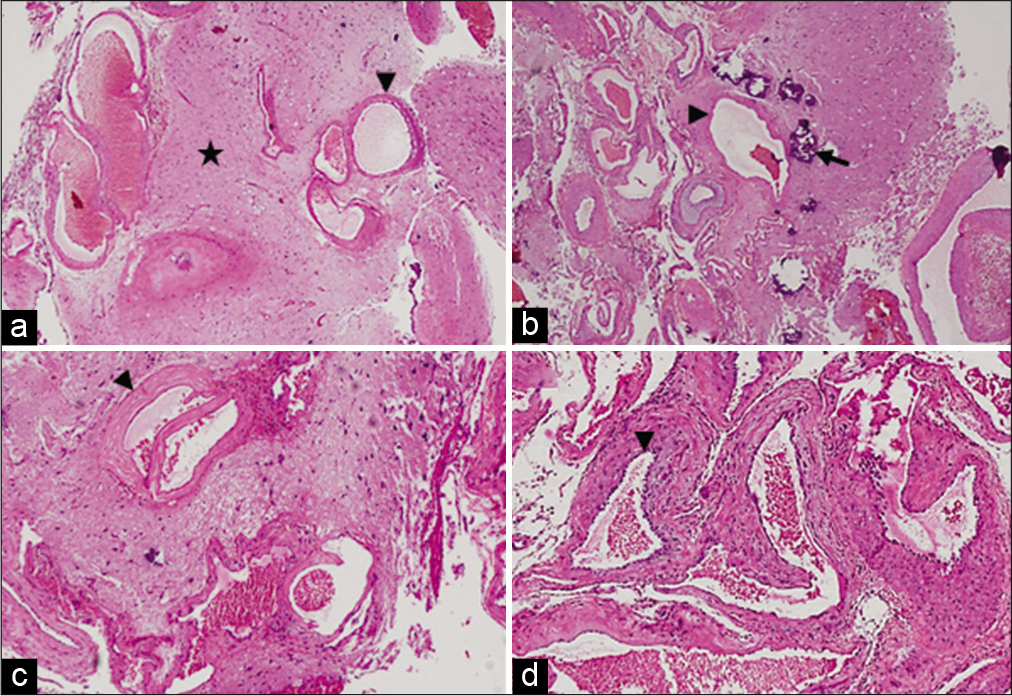
Figure 4:
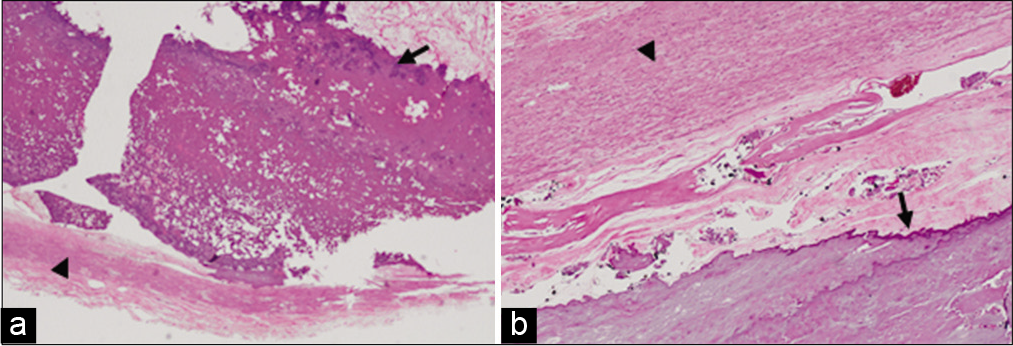
Photomicrographs of specimen sent: (a and b) H&E (×4) images showing normal glial tissue (black star) with malformed blood vessels. Calcified deposits are seen within the vessels. (c and d) H&E (×10) images showing abnormally dilated vessels with smooth muscle in the walls. Blood vessels are highlighted with black arrowheads. Similarly, black arrows indicate calcified deposits.
The patient remained stable postoperatively and was subsequently shifted to the intensive care unit for overnight monitoring. On the 1st postoperative day, he noticed difficulty in moving his left arm and leg against gravity. Power in his left upper extremity was 3/5 while power in his left lower extremity was 2/5. He did not have any additional neurological deficits. He was managed with intravenous fluids, antibiotics, anti-emetics, analgesics, and steroids. In addition, he received chest and limb physiotherapy and occupational therapy. A DSA on the 2nd postoperative day showed no evidence of residual disease or arterial blush [
DISCUSSION
While intracranial calcifications are not an uncommon finding in a variety of pathologies, what constitutes a “brain stone” or “cerebral calculus,” especially in terms of size and morphology, remains to be clearly determined. Nearly 60 years ago, Tiberin and Beller[
Brain stones can be classified based on their location, either extra-axial or intra-axial, and etiology. While extra-axial calcified lesions are usually tumors or exaggerated physiological calcifications, intra-axial lesions include vascular, neoplastic, congenital, infectious, endocrine, or metabolic anomalies.[
AVMs can lead to a variety of symptoms such as hemorrhagic stroke, seizures, headaches, and focal neurologic deficits. Asymptomatic AVMs may also be detected incidentally as noninvasive imaging modalities become more readily available and advanced.[
Cerebral angiography, using subtraction and magnification, remains the gold standard investigation for diagnosing AVMs. A CT scan can be used to initially image the AVM to detect ICH and calcifications. CTA provides further details about the location, size, and drainage of the AVM.[
At present, available treatment options for brain AVMs include microsurgical resection, stereotactic radiosurgery, and endovascular embolization, either performed as primary therapy or as part of a multimodal treatment plan. The goal of treatment is complete elimination of the nidus and arteriovenous shunt, as partial nidal obliteration carries a substantial risk of hemorrhage.[
This was our first experience of managing a patient with a calcified AVM. While the calcified nature of the lesion did not present a substantial increase in the difficulty of resection, we were faced with a diagnostic conundrum when the AVM was initially diagnosed as a neoplastic lesion. We were able to resect the lesion in its entirety, including the nidus, leading to improvement in the patient’s symptoms.
CONCLUSION
Large calcified intracranial lesions or brain stones are a rarely encountered pathology. Brain stones in association with various intracranial lesions have been seldom reported in the literature and even more infrequently in association with AVMs. We report the case of a pediatric patient with a large densely calcified AVM, who underwent successful microsurgical resection of the lesion.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Nil.
References
1. Al-Shahi R, Warlow C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001. 124: 1900-26
2. Barreau X, Marnat G, Gariel F, Dousset V. Intracranial arteriovenous malformations. Diagn Interv Imaging. 2014. 95: 1175-86
3. Brown RD, Wiebers DO, Forbes G, O’Fallon WM, Piepgras DG, Marsh WR. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988. 68: 352-7
4. Celli P, Ferrante L, Palma L, Cavedon G. Cerebral arteriovenous malformations in children. Clinical features and outcome of treatment in children and in adults. Surg Neurol. 1984. 22: 43-9
5. Celzo FG, Venstermans C, de Belder F, van Goethem J, van den Hauwe L, van der Zijden T. Brain stones revisited-between a rock and a hard place. Insights Imaging. 2013. 4: 625-35
6. Derdeyn CP, Zipfel GJ, Albuquerque FC, Cooke DL, Feldmann E, Sheehan JP. Management of brain arteriovenous malformations: A scientific statement for healthcare professionals from the American heart association/ American stroke association. Stroke. 2017. 48: e200-24
7. Florian IA, Popovici L, Timis TL, Florian IS, BerindanNeagoe I. Intracranial gorgon: Surgical case report of a large calcified brain arteriovenous malformation. Am J Case Rep. 2020. 21: e922872
8. Gezercan Y, Acik V, Çavuş G, Ökten AI, Bilgin E, Millet H. Six different extremely calcified lesions of the brain: Brain stones. Springerplus. 2016. 5: 1941
9. Gross BA, Frerichs KU, Du R. Sensitivity of CT angiography, T2-weighted MRI, and magnetic resonance angiography in detecting cerebral arteriovenous malformations and associated aneurysms. J Clin Neurosci. 2012. 19: 1093-5
10. Kahl W, Kessel G, Schwarz M, Voth D. Arterio-venous malformations in childhood: Clinical presentation, results after operative treatment and long-term follow-up. Neurosurg Rev. 1989. 12: 165-71
11. Laakso A, Hernesniemi J. Arteriovenous malformations: Epidemiology and clinical presentation. Neurosurg Clin N Am. 2012. 23: 1-6
12. Lawton MT, Rutledge WC, Kim H, Stapf C, Whitehead KJ, Li DY. Brain arteriovenous malformations. Nat Rev Dis Primers. 2015. 1: 15008
13. Leblanc R, Levesque M, Comair Y, Ethier R. Magnetic resonance imaging of cerebral arteriovenous malformations. Neurosurgery. 1987. 21: 15-20
14. Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: Aetiology, presentation and outcome. Brain Dev. 2003. 25: 416-21
15. Niazi TN, Klimo PJ, Anderson RC, Raffel C. Diagnosis and management of arteriovenous malformations in children. Neurosurg Clin N Am. 2010. 21: 443-56
16. Tiberin P, Beller AJ. Observations on so-called brain stones or cerebral calculi. Neurology. 1963. 13: 464-76
17. Yu YL, Chiu EK, Woo E, Chan FL, Lam WK, Huang CY. Dystrophic intracranial calcification: CT evidence of “cerebral steal” from arteriovenous malformation. Neuroradiology. 1987. 29: 519-22