- Department of Neurosurgery University of Florida, Gainesville, Florida, United States.
- Department of Pathology, University of Florida, Gainesville, Florida, United States.
Correspondence Address:
Lance S. Governale
Department of Neurosurgery University of Florida, Gainesville, Florida, United States.
DOI:10.25259/SNI_641_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dimitri Laurent1, Olgert Bardhi1, Jason Gregory2, Anthony Yachnis2, Lance S. Governale1. Pediatric pathology all grown up – An interesting case of adult tethered spinal cord. 29-Oct-2020;11:362
How to cite this URL: Dimitri Laurent1, Olgert Bardhi1, Jason Gregory2, Anthony Yachnis2, Lance S. Governale1. Pediatric pathology all grown up – An interesting case of adult tethered spinal cord. 29-Oct-2020;11:362. Available from: https://surgicalneurologyint.com/surgicalint-articles/10361/
Abstract
Background: Cervical myelopathy in an adult is typically the result of degenerative disease or trauma. Dysraphism is rarely the cause.
Case Description: The authors report the case of a 35-year-old male drywall installer who presented with 2 years of progressive left upper extremity weakness, numbness, and hand clumsiness. Only upon detailed questioning did he mention that he had neck surgery just after birth, but he did not know what was done. He then also reported that he routinely shaved a patch of lower back hair, but denied bowel, bladder, or lower extremity dysfunction. Magnetic resonance imaging of the cervical spine demonstrated T2 hyperintensity at C4-C5 with dorsal projection of the neural elements into the subcutaneous tissues concerning for a retethered cervical myelomeningocele. Lumbar imaging revealed a diastematomyelia at L4. He underwent surgical intervention for detethering and repaired of the cervical myelomeningocele. Four months postoperatively, he had almost complete resolution of symptoms, and imaging showed a satisfactory detethering. The diastematomyelia remained asymptomatic and is being observed.
Conclusion: Tethered cervical cord is a rare cause for myelopathy in the adult patient. In the symptomatic patient, surgical repair with detethering is indicated to prevent disease progression and often results in clinical improvement.
Keywords: Cervical myelomeningocele, Cervical myelopathy, Diastematomyelia, Spina bifida, Spinal dysraphism, Tethered spinal cord
INTRODUCTION
Cervical myelopathy in an adult is typically the result of degenerative disease or trauma.[
CASE REPORT
History and examination
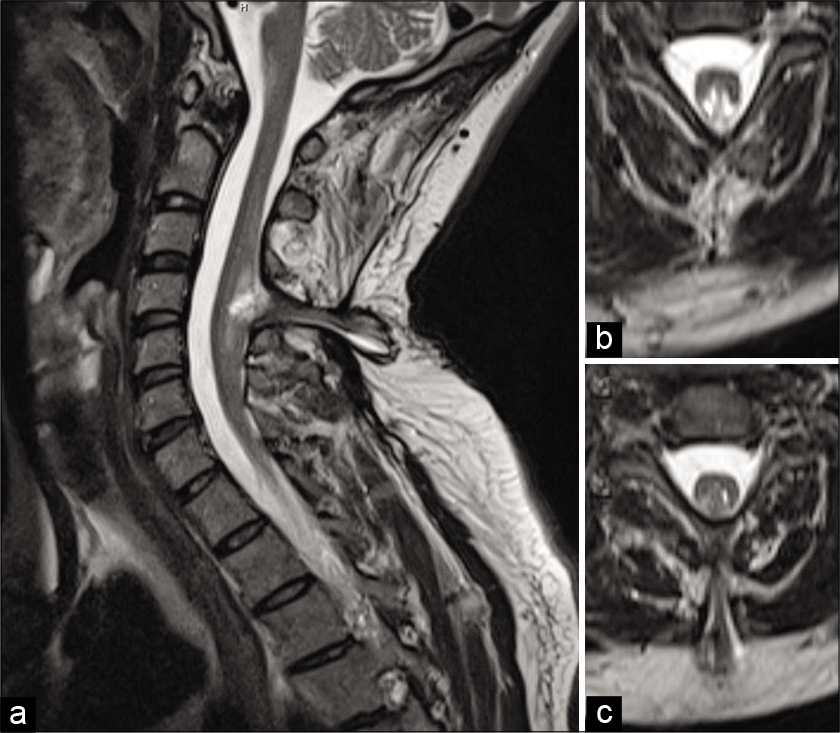
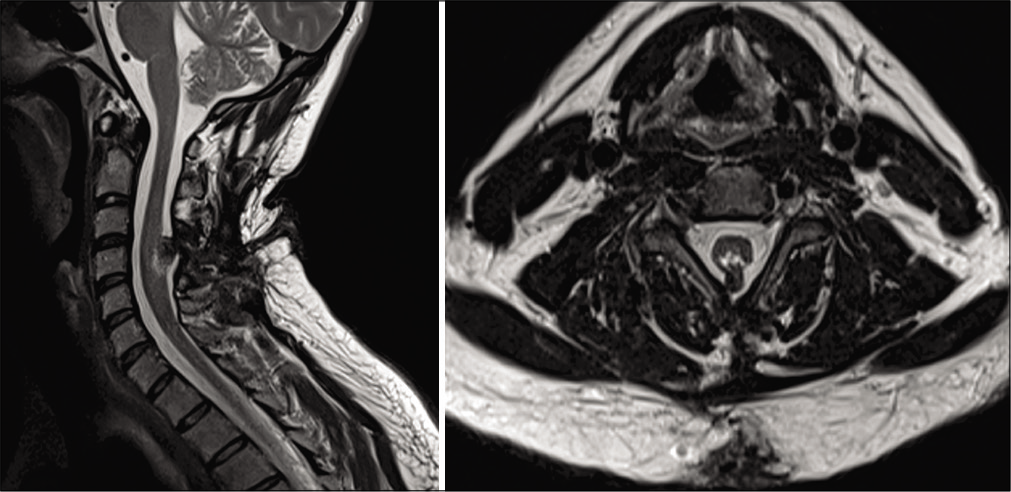
A 35-year-old male drywall installer presented with 2 years of progressive left upper extremity weakness, numbness, and hand clumsiness. Neurologic examination demonstrated 4-/5 strength of the left hand intrinsic muscles, decreased sensation to light touch in the left hand, and hyperreflexia of the left upper extremity. Electromyography and nerve conduction testing demonstrated only mild incidental bilateral ulnar neuropathy across the elbow. Magnetic resonance imaging (MRI) of the cervical spine demonstrated cervical spinal cord expansion with T2 hyperintensity at C4-C5. There was an associated defect of the bony posterior elements with projection of the neural elements into the dorsal subcutaneous soft tissue [
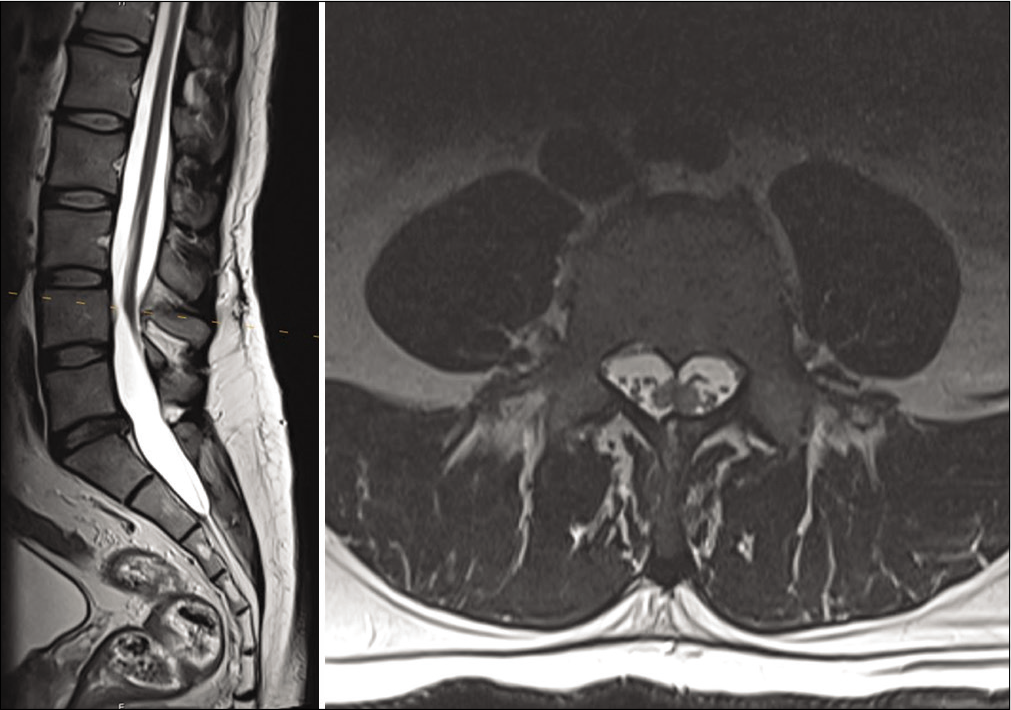
He then also reported that he routinely shaved a patch of lower back hair, but denied bowel, bladder, or lower extremity dysfunction. This prompted an MRI of the lumbar spine which demonstrated underlying diastematomyelia with a low-lying conus [
Operation
After satisfactory induction of general endotracheal anesthesia, the patient was positioned prone on gel rolls, and his head was fixed in Mayfield pins in neutral position. Neuromonitoring with somatosensory evoked potentials (SSEP), motor evoked potentials, and electromyography was established. The prior curvilinear midline incision was marked and slightly extended superiorly and inferiorly.
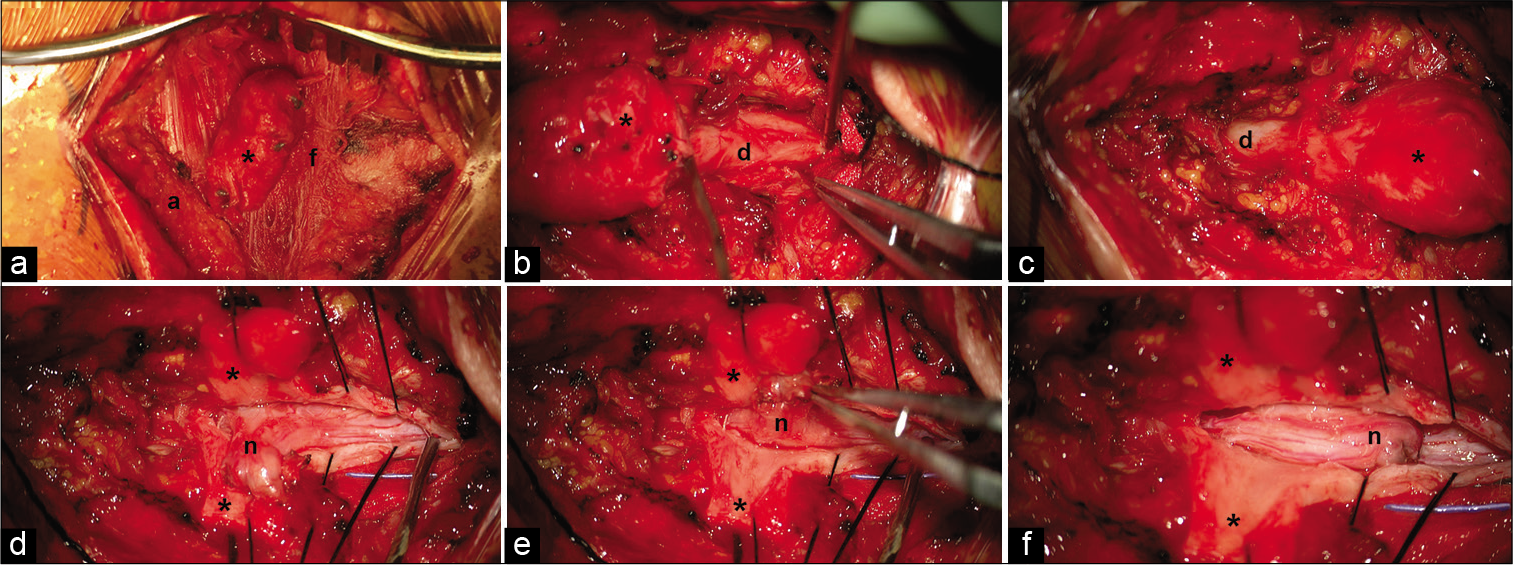
The superior portion of the incision over the last rostral intact lamina was opened sharply and carried down to the fascia using blunt dissection. Progressing along the fascia from superior to inferior, the overlying soft tissue was opened. The myelomeningocele sac was identified and dissected circumferentially as it passed through the fascia. The sac was then separated from the overlying soft tissue [
Figure 3:
Intraoperative views of the cervical myelomeningocele (cranial at right and caudal at left in each image). (a) Myelomeningocele sac (asterisk) extending through the fascia (f) and terminating in the subcutaneous adipose tissue (a). (b and c) Myelomeningocele emanating from the normal dura (d) and contiguous with it superiorly and inferiorly. (d) Dorsal projection of the neural elements (n) after dissection from the overlying myelomeningocele sac. (e) Pial closure of the dysplastic neural stump. (f) The free dysplastic neural stump at rest before dural closure.
The fascia was opened rostral and caudal to the myelomeningocele sac. The rostral (bifid) and caudal (hemi) dysplastic spinal lamina were removed using Kerrison rongeurs. The exposed native dura was contiguous with the myelomeningocele sac [
Under microscopic magnification, the spinal cord dura was incised rostral to the myelomeningocele. Progressing caudally, the dorsal spinal cord was found to be tethered to the dura by thick arachnoid bands. These were divided as they were encountered. The dural opening eventually extended into the myelomeningocele sac. In the sac was a dorsal projection of the cervical spinal cord approximately the same size as the cervical spinal cord. It was freed circumferentially from its dural attachments. The dorsal projection terminated in dysplastic spinal cord that fused with the overlying dural sac [
The remaining stump of dorsal projection still needed to be addressed. Sectioning the dorsal projection closer to the native spinal cord was avoided due to fear of transiting neural tracts within the projection. Left alone, the stalk came to a resting position along the dorsal surface of the rostral spinal cord [
The dura and overlying soft tissue were closed primarily in a watertight fashion. The neuromonitoring signals at the end of the operation were stable compared to the beginning. In fact, the left arm SSEP was mildly improved. There was no significant neuromonitoring signal change throughout the surgery.
Pathological findings
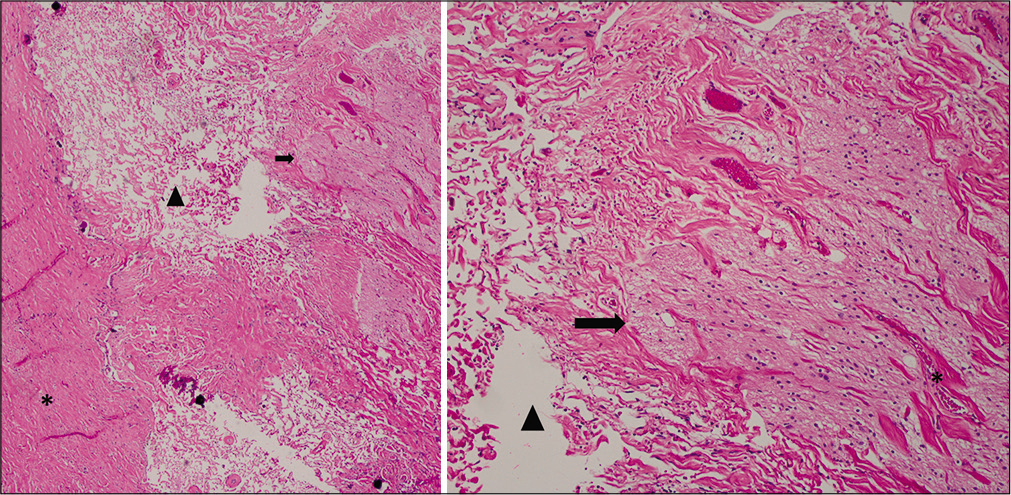
The dysplastic portion of the dorsal projection along with its fused overlying dura was examined [
Figure 4:
Hematoxylin and eosin staining of the myelomeningocele at the region of the neural-meningeal attachment. Left: low magnification study revealed fibrosis and collagen fiber bundles with prominent interfiber clefts (arrowhead), likely filled with cerebrospinal fluid. Dura is seen at left (asterisk), leptomeninges at center, and bands of neuroglial tissue (arrow) at right. Right: inspection at high magnification showed the clefts (arrowhead) between collagen fibers at left and bundles of neuroglial tissue (arrow) and meninges (asterisk) at right. Neuropil containing scattered oligodendrocytes with a dark nuclei and slight perinuclear halo is readily identified within the neuroglial tissue.
Postoperative course
On postoperative day 1, the patient reported improvement in his left upper extremity weakness and numbness. At 1 month follow-up, he reported continued improvement in his symptoms. He had some residual altered sensorium of the left hand, but improvement in his intrinsic hand muscle strength and no further pain, paresthesia, or clumsiness. He had returned to work without special accommodation. At 4 months follow-up, he had regained full strength in his left hand, and MRI showed a successful detethering without neural compression [
DISCUSSION
Cervical myelomeningoceles are a rare form of spinal dysraphism, accounting for <6% of all neural tube defects.[
Tethered spinal cord in adults most often presents with pain.[
There have been few reports of cervical myelomeningocele in the adult patient. Presentation may include pain, weakness, paresthesia, upper motor neuron signs, muscle wasting, and bowel/bladder dysfunction.[
Interestingly, our adult patient also had a previously undiagnosed lumbar diastematomyelia. Other than a patch of lower back hair that was routinely shaved, the lumbar diastematomyelia was asymptomatic. When these lesions are discovered in childhood, surgery is typically recommended to lessen the risk of developing tethering symptoms, which may be irreversible, overtime.[
CONCLUSION
Tethered cervical spinal cord is a rare cause for myelopathy in the adult patient. In the symptomatic patient, surgical repair with detethering is indicated to prevent disease progression and often results in clinical improvement. When asymptomatic, such as with this patient’s concurrent lumbar diastematomyelia, observation is prudent.
Declaration of patient consent
Patient’s consent not obtained as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abu-Bonsrah N, Purvis TE, Goodwin CR, Petteys RJ, de la Garza-Ramos R, Sciubba DM. Adult cervicothoracic lipomyelomeningocele. J Clin Neurosci. 2016. 32: 157-9
2. Balachandran G. Klippel-Feil syndrome and anterior cervical meningomyelocele: A rare case report. AJNR Am J Neuroradiol. 2009. 30: E130
3. Brokinkel B, Wiebe K, Hesselmann V, Filler TJ, Ewelt C, Muller-Hofstede C. Surgical treatment in a patient with Klippel-Feil syndrome and anterior cervical meningomyelocele: A case report and review of literature. Eur Spine J. 2013. 22: S517-20
4. Chen YC, Kuo CH, Cheng CM, Wu JC. Recent advances in the management of cervical spondylotic myelopathy: Bibliometric analysis and surgical perspectives. J Neurosurg Spine. 2019. 31: 299-309
5. Denaro L, Padoan A, Manara R, Gardiman M, Ciccarino P, d’Avella D. Cervical myelomeningocele in adulthood: Case report. Neurosurgery. 2008. 62: E1169-71
6. Eller TW, Bernstein LP, Rosenberg RS, McLone DG. Tethered cervical spinal cord. Case report. J Neurosurg. 1987. 67: 600-2
7. Gan YC, Sgouros S, Walsh AR, Hockley AD. Diastematomyelia in children: Treatment outcome and natural history of associated syringomyelia. Childs Nerv Syst. 2007. 23: 515-9
8. Habibi Z, Nejat F, Tajik P, Kazmi SS, Kajbafzadeh AM. Cervical myelomeningocele. Neurosurgery. 2006. 58: 1168-75
9. Iskandar BJ, Fulmer BB, Hadley MN, Oakes WJ. Congenital tethered spinal cord syndrome in adults. Neurosurg Focus. 2001. 10: e7
10. Klekamp J. Tethered cord syndrome in adults. J Neurosurg Spine. 2011. 15: 258-70
11. Meyer-Heim AD, Klein A, Boltshauser E. Cervical myelomeningocele-follow-up of five patients. Eur J paediatr Neurol. 2003. 7: 407-12
12. Perrini P, Scollato A, Guidi E, Benedetto N, Buccoliero AM, di Lorenzo N. Tethered cervical spinal cord due to a hamartomatous stalk in a young adult. Case report. J Neurosurg. 2005. 102: 244-7
13. Raheja A, Gupta DK, Nalwa A, Suri V, Sharma BS. Nonterminal cervical myelocystocele: Unusual cause of spastic quadriparesis in an adult. Neurol India. 2014. 62: 704-8
14. Rossi A, Piatelli G, Gandolfo C, Pavanello M, Hoffmann C, van Goethem JW. Spectrum of nonterminal myelocystoceles. Neurosurgery. 2006. 58: 509-15
15. Salomao JF, Cavalheiro S, Matushita H, Leibinger RD, Bellas AR, Vanazzi E. Cystic spinal dysraphism of the cervical and upper thoracic region. Childs Nerv Syst. 2006. 22: 234-42
16. Smith KA, Rekate HL. Delayed postoperative tethering of the cervical spinal cord. J Neurosurg. 1994. 81: 196-201
17. Yamada S, Zinke DE, Sanders D. Pathophysiology of tethered cord syndrome. J Neurosurg. 1981. 54: 494-503