- Department of Neurology and Neurosurgery, Stavropol State Medical University, Stavropol, Russian Federation
- Department of General Medicine, Stavropol State Medical University, Stavropol, Russian Federation
- Clinic of Neurosurgery, N.V. Sklifosovsky Research Institute for Emergency Medicine, Moscow Healthcare Department, Moscow, Russian Federation
- Biochemistry Lab, Stavropol Research Institute for Plague Control, Stavropol, Russian Federation.
- Turin, Italy
Correspondence Address:
Michael Lebenstein-Gumovski, Department of Neurology and Neurosurgery, Stavropol State Medical University, Stavropol, Russian Federation.
DOI:
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michael Lebenstein-Gumovski1, Alexander Zharchenko2, Tanzila Rasueva3, Robert Bashahanov2, Dmitry A. Kovalev4, Andrey Zhirov4, Anton Shatokhin1, Andrey Grin3. PEG-chitosan (Neuro-PEG) induced restoration of motor function after complete transection of the dorsal spinal cord in swine. A pilot study. 13-Dec-2023;14:424
How to cite this URL: Michael Lebenstein-Gumovski1, Alexander Zharchenko2, Tanzila Rasueva3, Robert Bashahanov2, Dmitry A. Kovalev4, Andrey Zhirov4, Anton Shatokhin1, Andrey Grin3. PEG-chitosan (Neuro-PEG) induced restoration of motor function after complete transection of the dorsal spinal cord in swine. A pilot study. 13-Dec-2023;14:424. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12666
Abstract
Background: Spinal cord injury (SCI) remains an unmet medical need. Recently, fusogens, such as polyethylene glycol (PEG), have been proven effective in restoring sensorimotor function after complete transection of the spinal cord at different levels and in different species. Here, we report on the use of a PEG-chitosan combo in a different animal model (swine).
Methods: Five Hungarian Mangalica pigs were subjected to complete transection of the thoracic cord (T7-9). Three animals were treated with locally injected PEG-chitosan (Neuro-PEG) gel; two acted as controls. PEG-600 was also injected intra- and post-operatively intravenously. Animals were submitted to rehabilitation, including electrical myostimulation. Results were assessed after 60 days using the Individual Limb Motor Score, the Porcine Thoracic Spinal Cord Injured Behavioral Scale, and the modified motor Basso, Beattie, and Bresnahan scale; sensory and sphincter functions were also assessed. Animals underwent in vivo spinal cord tracing with DiI. Immunofluorescence histology included NF-200, DAPI, and a fluorochrome-conjugated secondary antibody.
Results: Starting on postoperative day (POD) 2, neuro-PEG-treated animals evinced the first signs of recovery, and on POD 60, they could all support their weight and were mobile. Controls never recovered any useful function. Fluorescence microscopy in the experimental group revealed axons passing through the site of injury, while degenerative post-traumatic changes were noted in controls.
Conclusion: Neuro-PEG affords sensorimotor recovery after complete spinal cord transection. This opens the door to human experimentation, including trials of spinal cord transplantation.
Keywords: Chitosan, Fusogens, Fusogen-sealants, Neuro-PEG, Polyethylene glycol, PEG-chitosan, Spinal cord injury
INTRODUCTION
Spinal cord injury (SCI) followed by permanent paralysis represents an unmet clinical challenge.[
Starting in 2013,[
In view of these results, our team developed a conjugate based on PEG and chitosan (PEG-chitosan or NeuroPEG), which showed its effectiveness in rodent and lagomorph models.[
MATERIALS AND METHODS
We used Chitosan with a molecular weight of 15 kDa (Merck, Germany), stabilized by photo-crosslinking, in a homogeneous mixture with PEG-600, (Merck, Germany), at a concentration of 20 mg/mL. The resulting mixture was sterilized and stored in sealed tubes at a temperature of 4°C (Neuro-PEG).
After obtaining institutional approval from the Local Ethics Committee, female pigs of the Hungarian Mangalitsa breed (m = 20.0 ± 2.0 kg, n = 5) were used (three experimental and two controls). All animals were treated in accordance with the European Convention for the Protection of Vertebrate Animals used for experiments or other scientific purposes. The animals were anesthetized with a mixture of Zoletil (Virbac, France) and Xylazine (Alfasan Int., Netherlands) intramuscularly, followed by propofol (PropofolKabi - Fresenius Kabi, Germany) through a central venous catheter. The animals were then paralyzed and intubated (air-oxygen mixture); a urethral urinary catheter was inserted.
Surgery
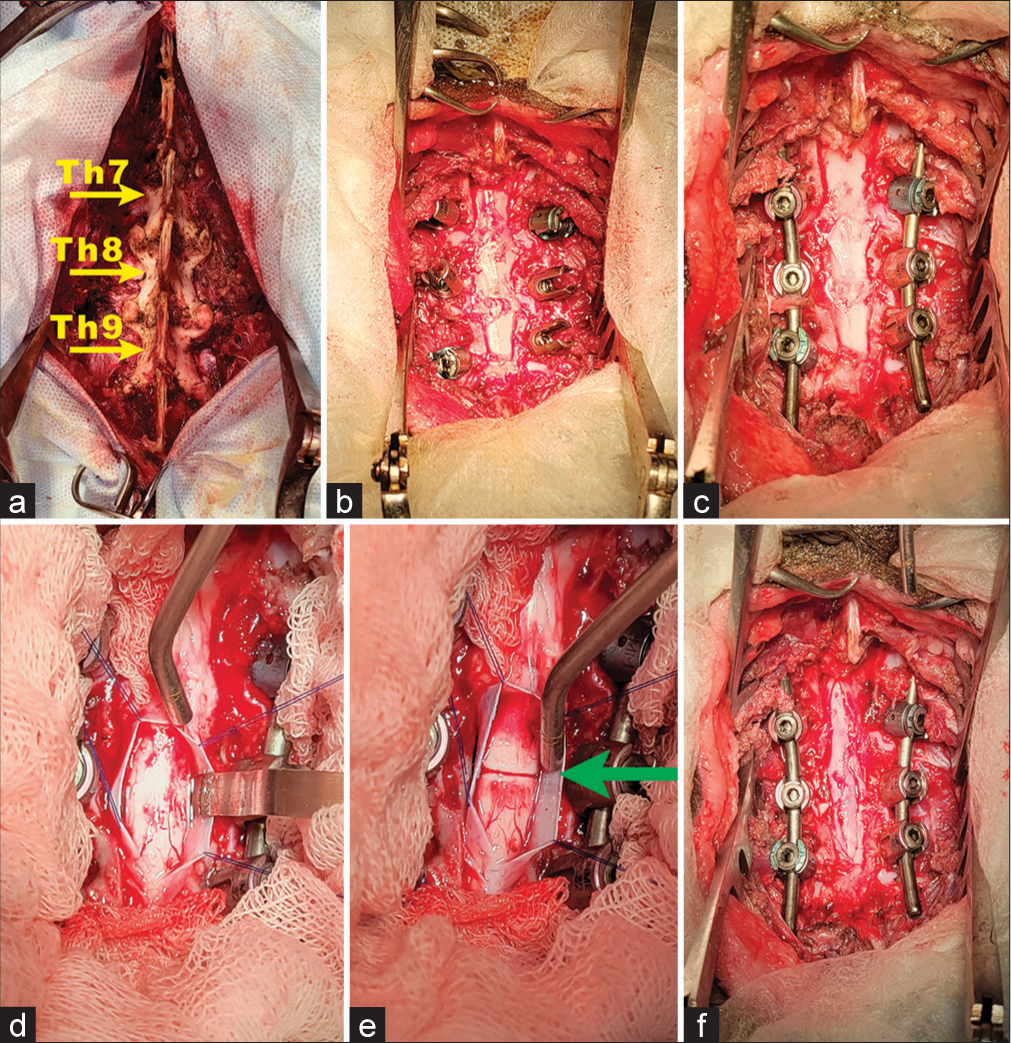
A T7-9 extended laminectomy was performed in standard fashion. The T7-9 segment was then stabilized with screws and rods: dorsal traction was applied. After that, a Smith-Peterson subtraction spondylotomy was performed. Ice slurry from a frozen isotonic sodium chloride solution (t = 0±0.5°С) was placed on the dural sac for 1 min. The dura mater was then opened longitudinally and fixed laterally. A flexible spatula was placed under the spinal cord to protect the dura, and the cord was fully transected with a No. 11 scalpel. The resulting diastatic space was flushed with 2 mL of neuro-PEG. In parallel, an intravenous drip infusion of 50 mL of PEG 600 solution was performed. Control animals (n = 2) were injected with saline. Water-tight sutures were applied to the dura, and the suture itself was covered with fatty tissue. After careful hemostasis of the wound, a vacuum drain was installed. The wound was sutured in layers and covered with an aerosol bandage [
Figure 1:
Operative steps. (a) Skeletonization of the vertebral arches of T7, T8, and T9 (Yellow arrows); (b) laminectomy and access to the spinal cord. Pedicle screws are installed; (c) the longitudinal rods are installed with the traction of the screws; (d) the dura mater is dissected, and lifted on thread-holders; a spatula is placed under the spinal cord; (e) axial section of the spinal cord (green arrow); and (f) dura closure performed [cranial end on top].
Postoperative rehabilitation
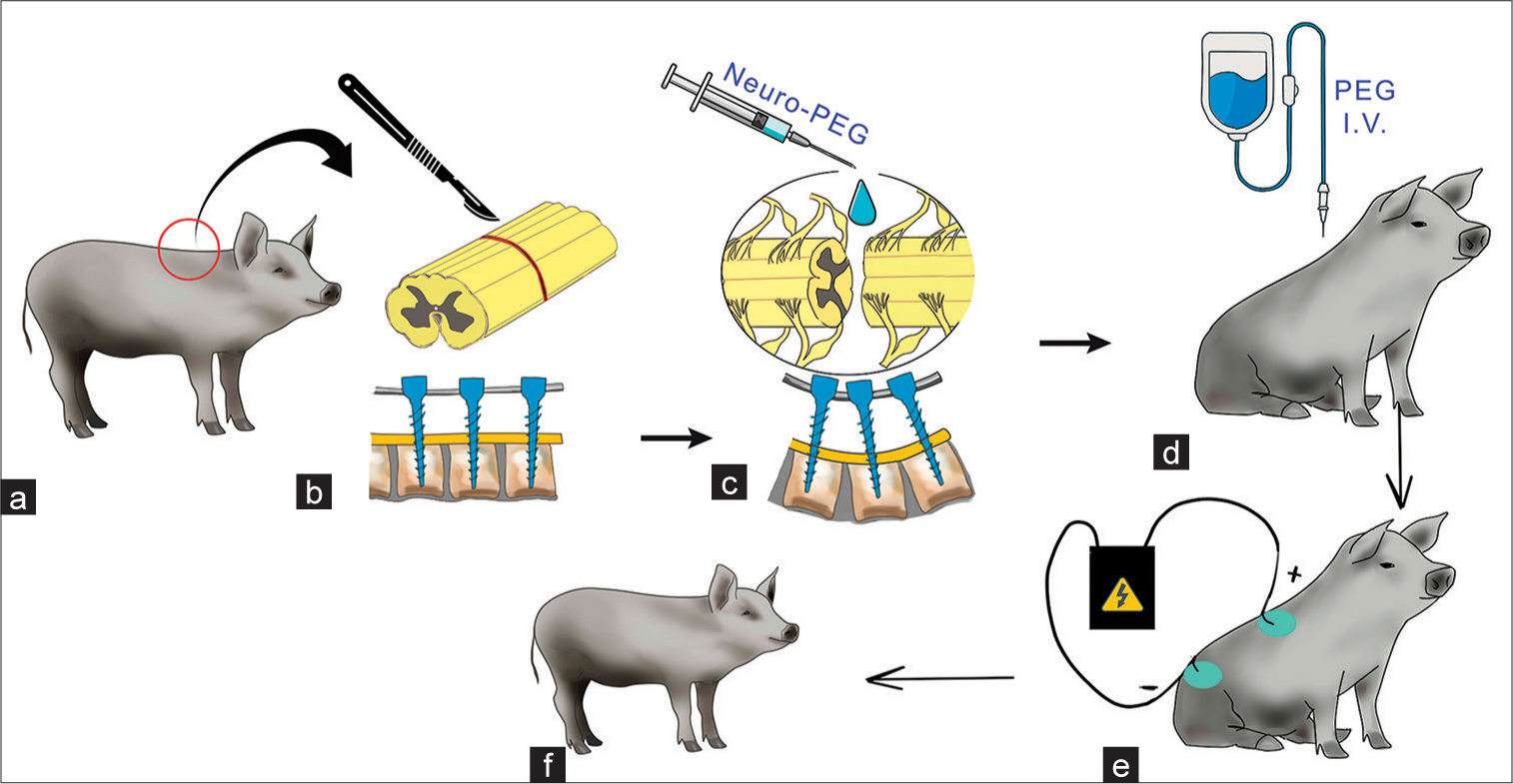
Post-operatively, antibiotics and analgesics were administered. A day-night regime was instituted with free access to water and regulated nutrition. The premises were air-conditioned, and constant temperature was maintained. The animals underwent daily massage of the hind limbs to improve trophism and prevent bedsores. Daily electromyostimulation was also used on the limbs (3–13 Hz, PW 0.1–30 ms, 20 min/ limb, bid) (experimental electromyostimulator “TiT,” LLC «TiT», Russia). Subcutaneous injections of neostigmine methyl sulfate (18 mcg/kg twice daily) and intramuscular injections of trypsin (10 mg once daily) were given for 15 days. For seven days, the three experimental animals were injected with IV PEG 600 (50 mL of a 25% solution of standard saline), qid. During rehabilitation, animals were placed on all four limbs with pelvic support. The urethral catheter was only removed after restoration of urination, as assessed every three days after test removal of the catheter for six hours. Defecation deficits were countered with enemas [
Figure 2:
Design of the experiment. (a and b) transection of the spinal cord at the thoracic level; installation of pedicle screws; (c) irrigation of the sectional surfaces of the spinal cord stumps with Neuro-PEG; vertebral stabilization with pedicle screws; (d) IV infusion of PEG in the postoperative period; (e) neurorehabilitation, including electrical myostimulation; and (f) early verticalization of animals after surgery.
Neurologic assessment
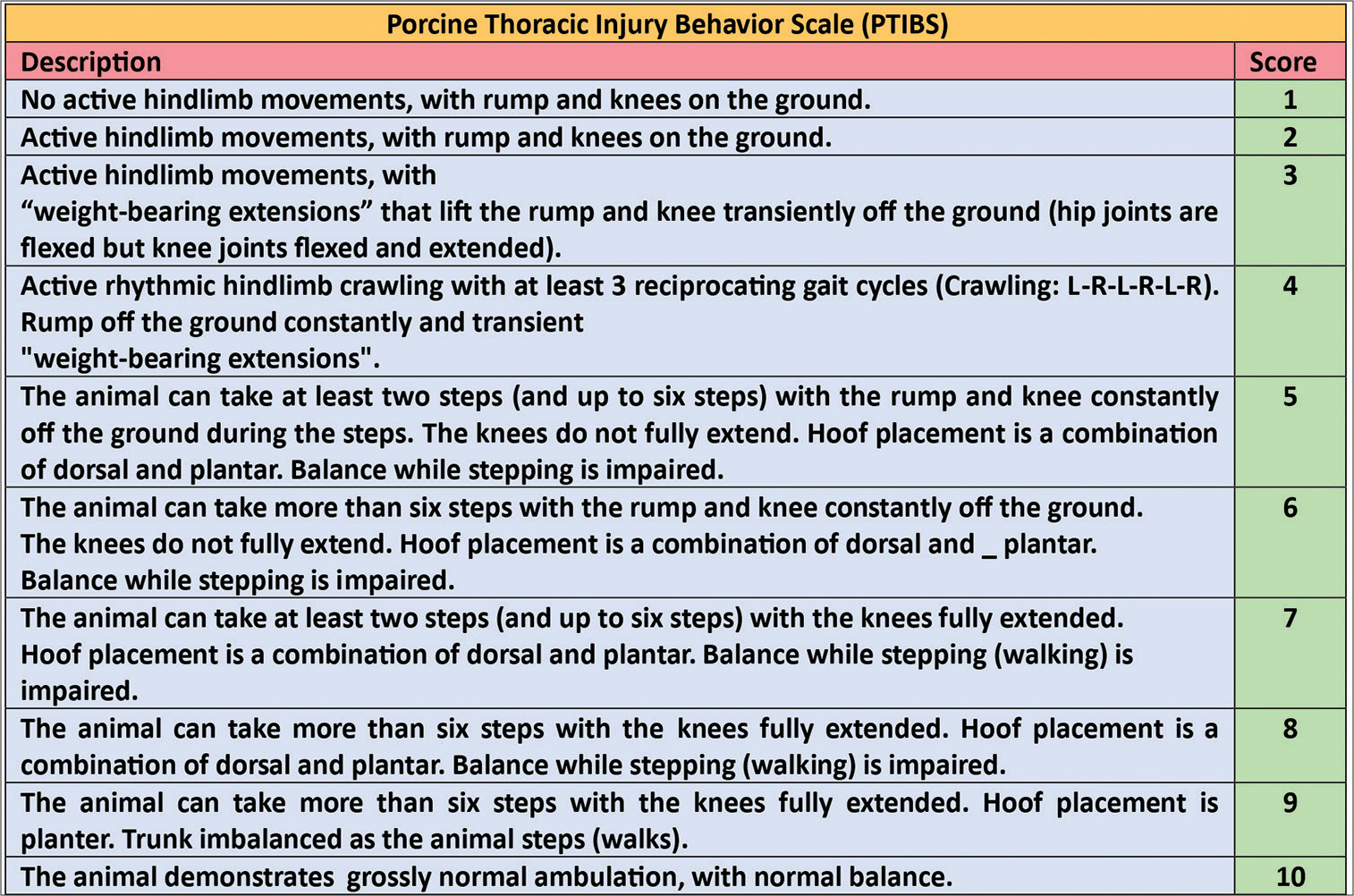
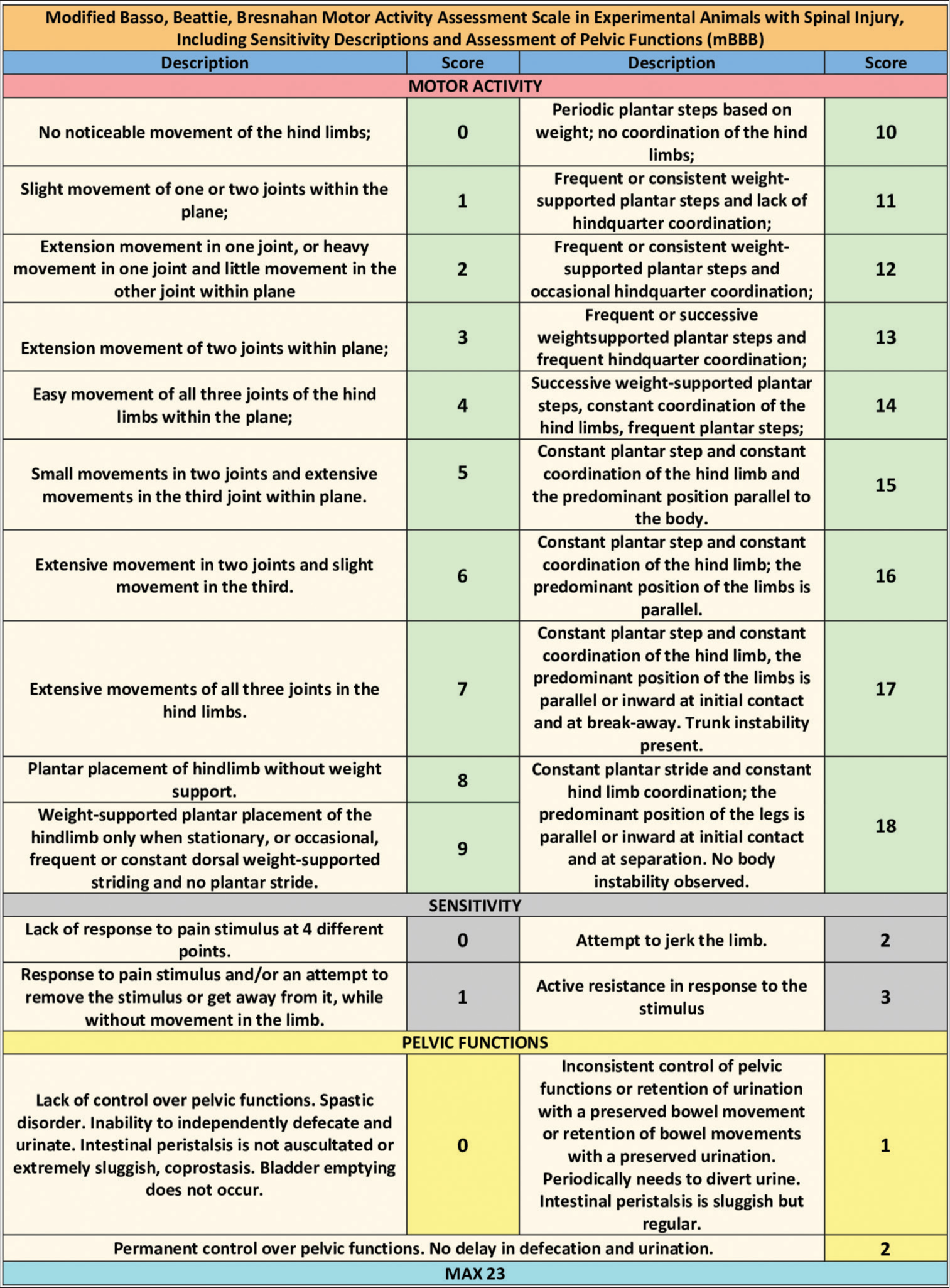
Neurologic assessment (motor) was carried out using the Individual Limb Motor Score [
Immunofluorescence
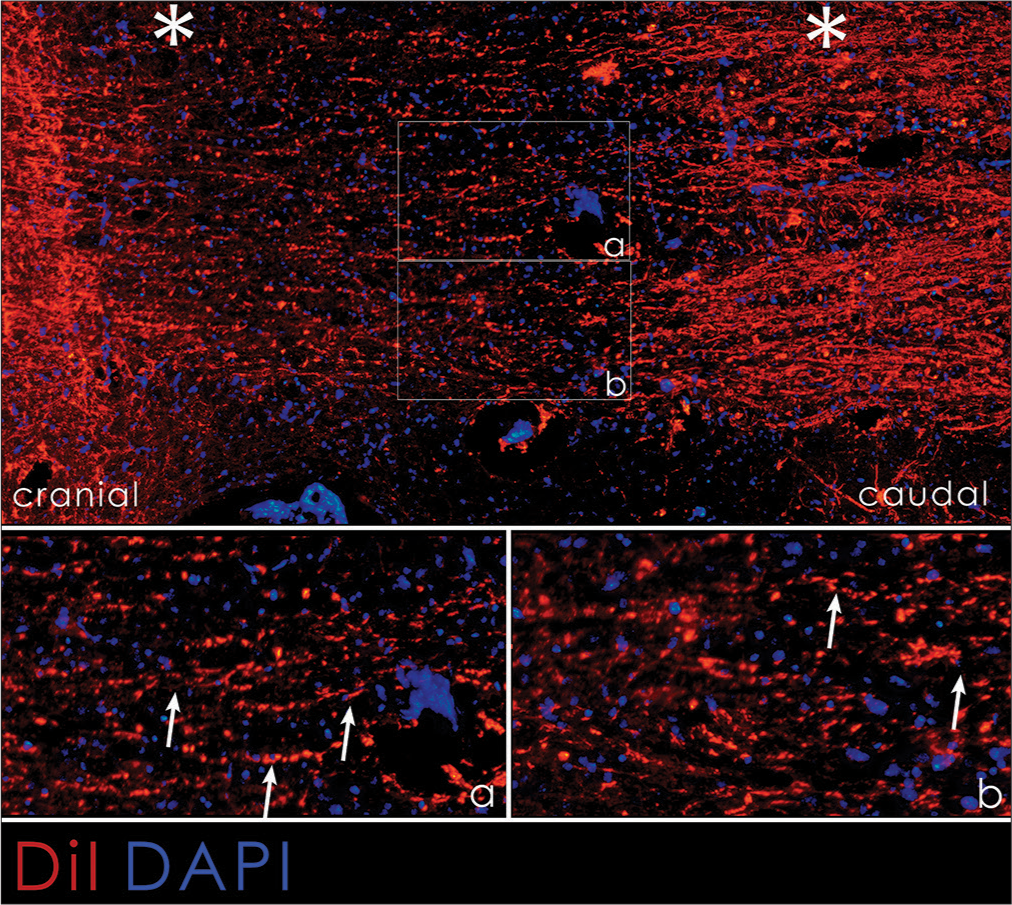
After induction of anesthesia with Zoletil, on post-operative day (POD) 50, microinjections of 5 μL of a 2% solution of 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine (DiI) (Abcam, UK) in dimethyl sulfoxide - (DMSO, Merck, Germany), were performed into the spinal cord 3 cm above the injury site, using a Hamilton syringe for 5 min. On day 61, all animals were euthanized with an overdose of Zoletil and Xylazine; a 10-cm section of the spinal cord, including the area of injury, was removed and immediately fixed in a 4% solution of paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) with pH 7.4. The samples were cryoprotected in 20% sucrose at 4 °C for 48 h, and the frozen samples were cut into longitudinal horizontal sections 30–35 μm thick using a cryotome. For permeabilization, slides with sections were immersed in Tween-20 0.3% (Abcam, UK) for 20 min. Blocking of nonspecific binding was carried out in 1% FGBA (gelatin solution ×10 Fish Gelatin Blocking Agent, Biotium, USA). Afterwards, a dilution of monoclonal mouse primary antibodies against neurofilament 200 (NF-200) (monoclonal mouse antibodies Anti-200 kD, NF-200 – Neuronal Marker, Abcam, UK) was prepared in 1% FGBA, and slides were incubated in this solution for one hour at room temperature. Dilutions of secondary antibodies conjugated with Alexa Fluor 488 fluorochrome (Rat monoclonal [SB84a] Anti-Mouse IgG2a heavy chain secondary antibodies [Alexa Fluor® 488], Abcam, UK) were also prepared with 1% FGBA. Incubation with secondary antibodies was carried out in the dark for one hour. Cell nuclei were stained with a 300 nM solution of 4’,6-diamidino-2-phenylindole – DAPI (Abcam, UK) in PBS. The slides were allowed to air-dry, and a drop of mounting medium was added. The specificity of staining was assessed by excluding primary antibodies or by incubation with FGBA. Fluorescence microscopy was performed using an AxioScope A1 microscope (Zeiss, Germany), a BioTek Cytation-1 multimode cell imaging module (Agilent Technologies BioTek, USA), and BioTek Gen5 software (Agilent Technologies BioTek, USA) with appropriate filters. The sections were studied and micrographed. Photo processing was carried out using ImageJ software (NIH, USA).
Statistical analysis
One-way analysis of variance and Spearman correlation tests were used to assess for significance for all data sets (threshold for significance: P ≤ 0.01)
RESULTS
Neurologic recovery
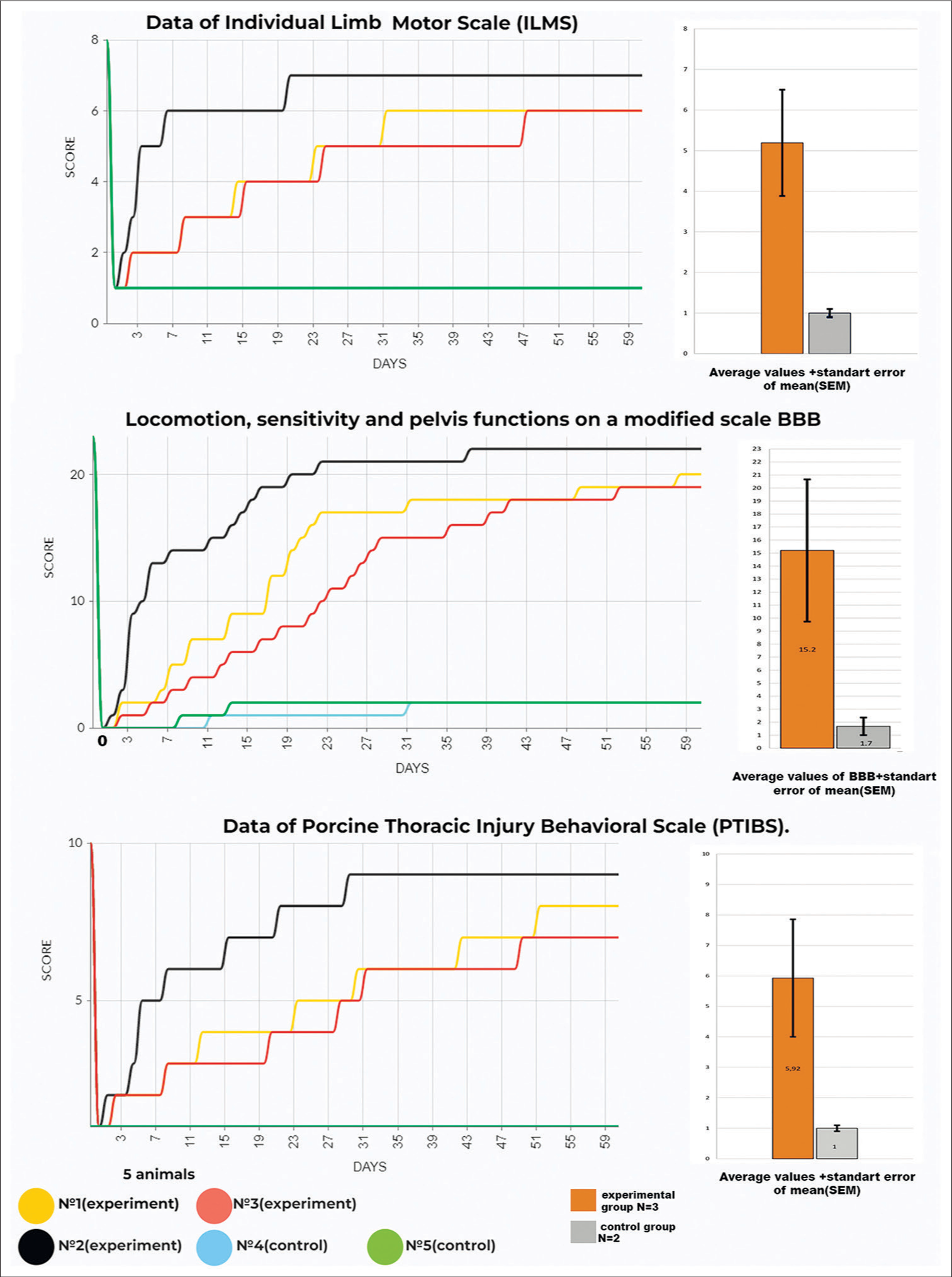
Post-operatively, all animals evinced paraplegia, anesthesia of the lower half of the body and sphincter incontinence. Coprostasis and urinary retention were equally pronounced up to post-operative day (POD) 2 in both groups. Subsequently, controls showed no signs of recovery over 60 days. On the other hand, experimental animals showed signs of recovery from POD 1. One animal evinced clear movements of the hind paws on the horizontal plane (weak pulling of the paws under active abduction by the experimenter). On POD 2, it reacted to a needle prick in three out of six control points on the skin of the hind limbs, and by POD 4, sphincter (urinary and bowel) control was restored. The other two animals had their catheters removed on POD 10. On POD 6, one treated animal showed active attempts to stand upright on its hind limbs. By POD 6, the strength in the hind limbs of two animals was sufficient to push off and counter pressure from the experimenter’s hand; one animal independently made active movements with its hind limbs but without attempts to stand. On POD 9, one animal could stand up freely and attempt to walk using its hind limbs [
Figure 6:
Recovery of motor function. 1st row from top: Postoperative day (POD) 6. The animal makes active movements with its hind limbs but without attempts to stand upright. 2nd row from top: POD 6. In one treated animal, attempts to stand upright using the hind limbs were noted. 3rd row from top: POD 9. One treated animal showed the ability to stand upright freely and attempted to walk using its hind limbs. 4th row from top: by POD 21, the animal stands confidently on all limbs, with full support; the range of motion has increased significantly; some instability when walking is visible. 5th row from top: On POD 55, the animal walked independently in the enclosure on all four limbs.
Figure 7:
Motor activity scores of Individual Limb Motor Score, modified Basso, Beattie, and Bresnahan (BBB), and Porcine Thoracic Spinal Cord Injured Behavioral Scale (No. 1–3 – experimental animals, No. 4 and 5 – controls)—right side: bar charts of means. Data in graphs are shown as means ± standard error of the mean.
Video 1
All results were statistically significant on all applied scales at POD 60 (P = 0.001; Spearman Correlation Coefficient: 0.726).
Immunofluorescent staining
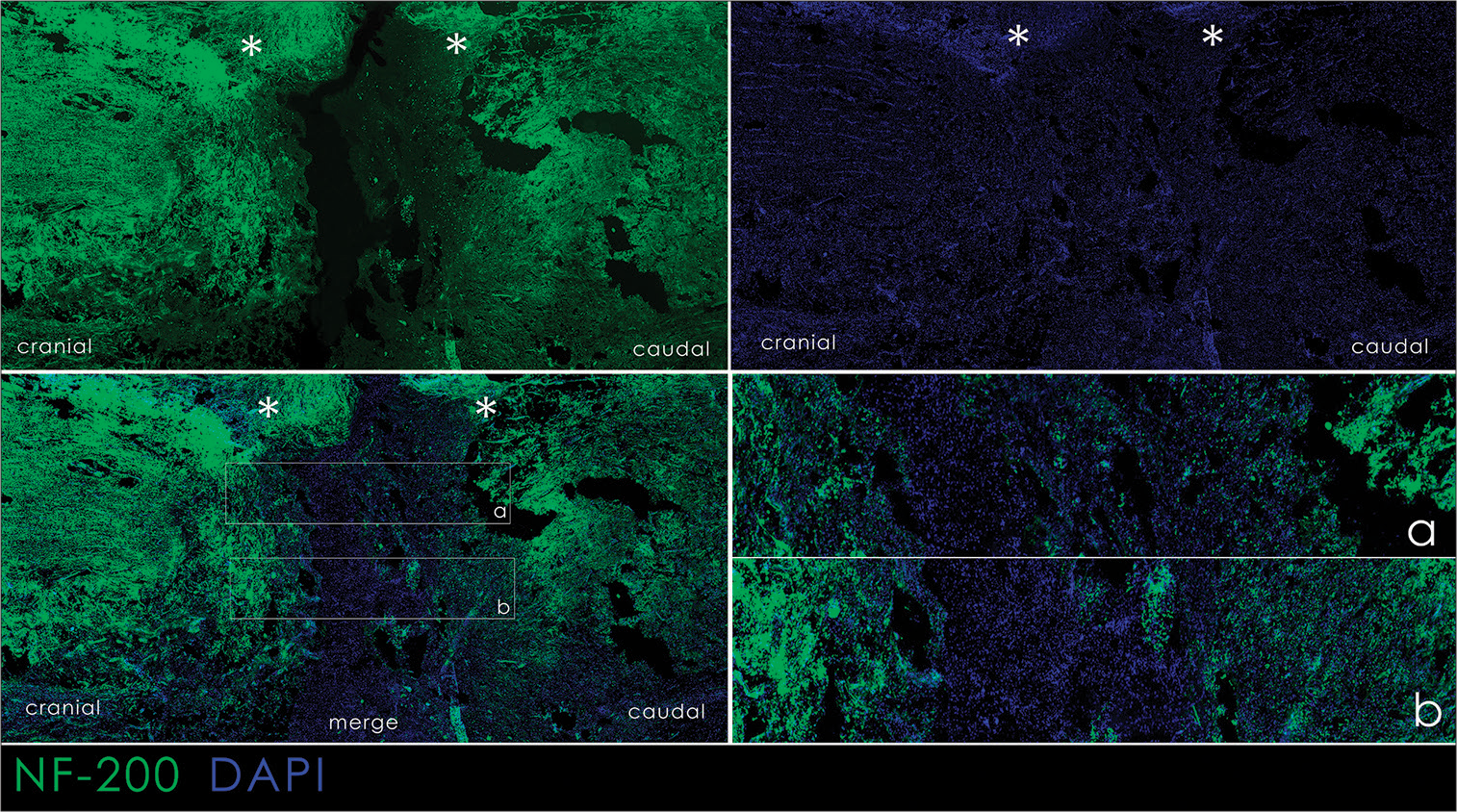
In controls, the site of transection was clearly visible, with axonal loss in areas undergoing Wallerian degeneration. A decrease in the amount of NF-200 in the caudal segment of the spinal cord was noted as a sign of axonal Wallerian degeneration. In the area of injury, loose tissue was observed devoid of NF-200 staining; an area of glial degeneration and scarring diffusely stained for DAPI, a nuclear dye [
Figure 9:
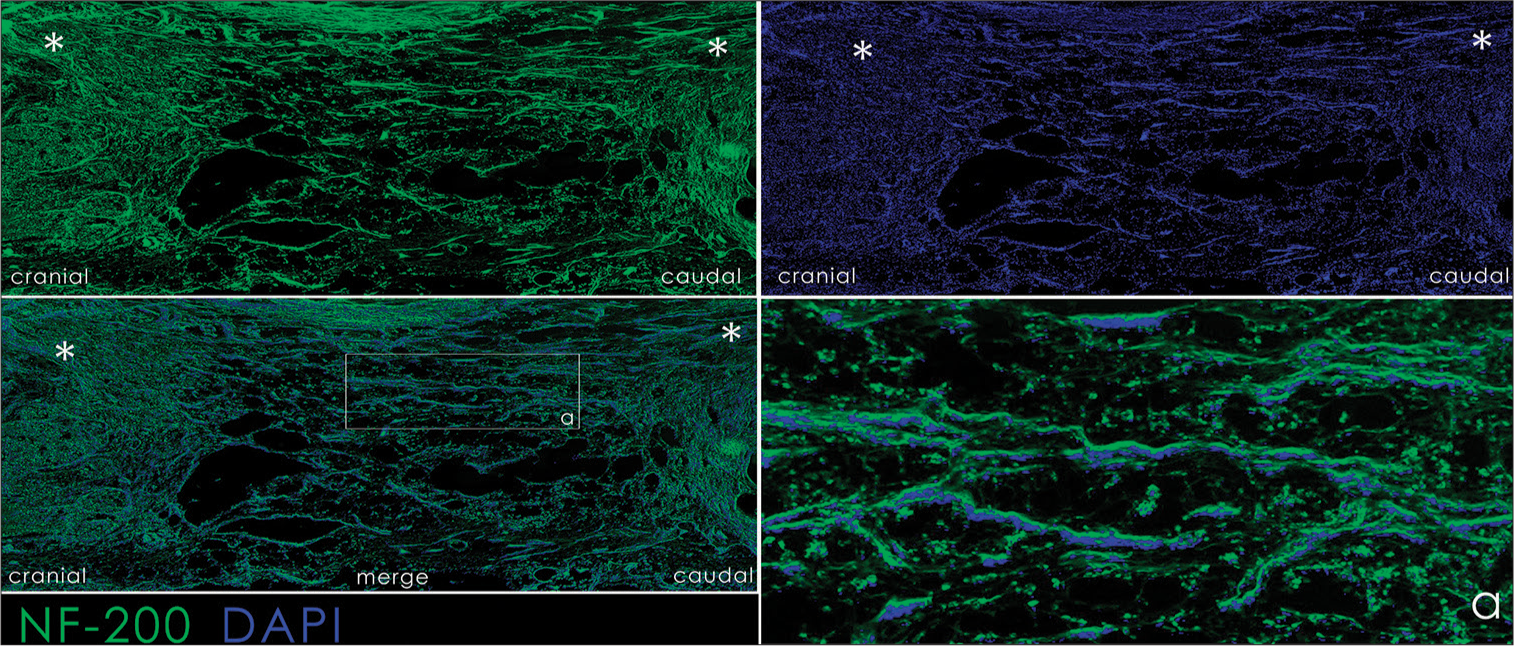
Immunofluorescence histology of the spinal cord of experimental animals. DAPI staining and staining for neurofilament-200; a combination of DAPI+NF-200; a: area of transection. The horizontal section; cranial end are on the left. Asterisks indicate the distal and proximal ends of the spinal cord.
Figure 10:
DiI immunofluorescence histology of the spinal cord of treated animals. Combination of DAPI+DiI; (a and b) area of spinal cord injury. The horizontal section; cranial end are on the left. Asterisks indicate the distal and proximal ends of the spinal cord. White arrows indicate the axons crossing the intersection site.
DISCUSSION
In this study, we show that fusogens can reverse spinal paralysis after complete transection in a large animal model. Although the study is small, the results are clear-cut.
The previous studies of PEG application in a similar experimental scenario were highly positive,[
Fusogens are part of the GEMINI spinal cord fusion protocol,[
We notice that our approach includes not only immediate local application of a fusogenic mixture but also intravenous injection of PEG over three days, plus local cord cooling as an adjunct neuroprotective measure. To keep the spinal stumps apposed for fusion and avoid displacements, we employed vertebral stabilization for the first time in fusogen SCI studies. Finally, electrical muscular stimulation, along with massage and motivational prompts, complement the method—the contribution of all these components on their own remains to be quantified.
CONCLUSION
We present the results in a large animal model of the use of our proprietary fusogenic PEG/Chitosan combination after full spinal cord transection. No other intervention deployed over the past 30 years in similar SCI models reached the levels of recovery seen here.[
Ethical approval
The study protocol was approved by the Local Ethics Committee of the Stavropol State Medical University (No. 97, April 15, 2021). This trial was carried out in accordance with the ethical standards of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes. The author(s) declare that they have taken the ethical approval from IRB.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12: 1-21
2. Canavero S, Ren X, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI). Surgery. 2016. 160: 11-9
3. Canavero S, Ren X, Kim CY. Heterologous spinal cord transplantation in man. Surg Neurol Int. 2021. 12: 295
4. Canavero S. Heaven: The head anastomosis venture project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int. 2013. 4: S335-42
5. Canavero S The. “Gemini” spinal cord fusion protocol: Reloaded. Surg Neurol Int. 2015. 6: 18
6. Canavero S, editors. The technology of brain transplantation. United States: Amazon IP; 2020. p.
7. Canavero S. Whole brain transplantation in man: Technically feasible. Surg Neurol Int. 2022. 13: 594
8. Cerro PD, Barriga-Martín A, Vara H, Romero-Muñoz LM, Rodríguez-De-Lope Á, Collazos-Castro JE. Neuropathological and motor impairments after incomplete cervical spinal cord injury in pigs. J Neurotrauma. 2021. 38: 2956-77
9. Kathe C, Skinnider MA, Hutson TH, Regazzi N, Gautier M, Demesmaeker R. The neurons that restore walking after paralysis. Nature. 2022. 611: 540-7
10. Kim CY, Baek J, Canavero S, Ren X, editors. Spinal cord fusion in rodents and dogs. The technology of head transplantation. New York: NOVA Science; 2020. p. 83-92
11. Lebenstein-Gumovski MV, Botasheva VS, Kovalev DA, Shirov AM, Shatokhin AA, Shatokhin AV. Method for restoring the functions of the spinal cord after transection thereof, using PEG-chitosan conjugate, Russian Federation patent RU 2782119 C1, registration № 2021120198. 2021. p.
12. Lebenstein-Gumovski MV, Grin’ AA, Kovalev DA, Bashakhanov RM, Rasueva TS, Zharchenko AV. Method of treatment of spinal cord injury with restoration of its functions with PEG-chitosan conjugate «NEURO-PEG», Russian Federation patent RU 2801469 C1, registration № 2022128384. 2022. p.
13. Lebenstein-Gumovski MV, Bashakhanov RM, Kovalev DA, Zhirov AM, Shatohkin AA, Botasheva VS. Recovery of spinal cord functions after experimental complete crossection under the effect of chitosan polymeric compounds. Zh Vopr Neirokhir Im N N Burdenko. 2023. 87: 36-44 (In Russ)
14. Lebenstein-Gumovski MV, Botasheva VS, Kovalev DA, Shatokhin AA. Characterization of morphological and functional changes in the spinal cord after its section, under the influence of a hydrogel based on a modification of chitosan. Volgogradskii Nauchno Med Z. 2021. 3: 37-42 (In Russ)
15. Lee JH, Jones CF, Okon EB, Anderson L, Tigchelaar S, Kooner P. A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma. 2013. 30: 142-59
16. Lorach H, Galvez A, Spagnolo V, Martel F, Karakas S, Intering N. Walking naturally after spinal cord injury using a brain-spine interface. Nature. 2023. 618: 126-33
17. Ren X, Kim CY, Canavero S. Bridging the gap: Spinal cord fusion as a treatment of chronic spinal cord injury. Surg Neurol Int. 2019. 10: 51
18. Ren X, Li M, Zhao X, Liu Z, Ren S, Zhang X, Canavero S, Ren X, editors. Fusogens: The engine of recovery. The technology of head transplantation. New York: NOVA Science; 2020. p. 69-81
19. Ren X, Li M, Zhao X, Liu Z, Ren S, Zhang X, Canavero S, Ren X, editors. Spinal cord fusion in primates. The technology of head transplantation. New York: NOVA Science; 2020. p. 93-101
20. Ryan I, Henderson PW, Canavero S, Ren X, editors. Gemini: Overview of fusogens. The technology of head transplantation. New York: NOVA Science; 2020. p. 51-68
In 1974, it was discovered how PEG evinced fusogenic properties on cell membranes. In 1981, Bittner first reported how single axons submitted to sharp transection could be restored (“refused”) by applying PEG and in 1999, Borgens’s group first reported on the capacity of PEG to refuse the axonal component of a severed spinal cord in guinea pigs. [
This changed in 2013 with the introduction of the GEMINI spinal cord fusion protocol, a combination of topically applied fusogens (e.g., PEG) to transected cords combined with spinal cord stimulation – SCS.[
Unfortunately, the SCI community as a whole spurned this approach (perhaps the prospect of having their funds rescinded bears some relevance here!), and basically, only the HEAVEN team and a few others pursued this approach in further animal studies with the goal of treating SCI. Recently, a group confirmed the efficacy of combined PEG and SCS in rodents.[
Chitosan, as reported by Lebenstein-Gumovski et al, is another remarkable fusogen. A combination of PEG and Chitosan makes sense, and in fact, studies of PEG and photo-cross-linkable chitosan have been published in the past.[
What needs discussion is the overall approach taken by the authors. PEG was also injected IV to maximize effects. Actually, studies of IV PEG in SCI dogs had mixed results [
These authors applied topical hypothermia to the damaged cord with the aim of neuroprotection. Spinal cord hypothermia was first applied decades ago and is still being assessed clinically. [
The GEMINI protocol includes electrical stimulation (SCS, invasive or non-invasive, plus transcranial magnetic stimulation). In the present study, myostimulation was administered, which makes sense. Rehabilitation was also instituted. These authors should be commended for the combined approach.
How do locally injected fusogens work? We now know that the fusion of axons in the white matter only plays a secondary role, as motor fibers are not neatly distributed in bundles but, in real life, tend to be more widespread. For PEG fusion to work, one needs the exact same axon to be aligned without too much pressure for several minutes to achieve fusion, and one sees how this is clearly impossible to achieve. Instead, spinal cord fusion exploits one of GEMINI’s principles, that is, the neuroprotectant effect on the gray matter (motor highway [
We do not know whether the combination of PEG and chitosan is superior to either one, or these calls for ad hoc studies: teasing out the effects of each and possible synergistic effects is paramount. Parenthetically, we notice that Bittner’s group recently reported that their fusion protocol is not superior to PEG alone.[
Another important point is that central pain was not reported by the authors, which is in line with all previous studies,[
Finally, PEG’s potential to induce allergic reactions, including anaphylaxis, is associated with very high molecular weights, not PEG600, as used by these and other authors.[
In conclusion, I commend this Russian team for a meticulously conducted study in a large animal SCI model of a fusogenic combination. This small trial was conducted with a human trial in sight and is all the more informative given the highly relevant results. One can only wonder whether this study will finally convince SCI associations to sponsor a trial of this sort.
Sergio Canavero, MD
Turin, Italy
References
1. Amoozgar Z, Rickett T, Park J, Tuchek C, Shi R, Yeo Y. Semi-interpenetrating network of polyethylene glycol and photocrosslinkable chitosan as an in-situ-forming nerve adhesive. Acta Biomater. 2012. 8: 1849-58
2. Batchelor P, Bernard S, Gantner D, Udy A, Board J, Fitzgerald M. Immediate cooling and early decompression for the treatment of cervical spinal cord injury: A safety and feasibility study. Ther Hypothermia Temp Manag. 2023. 13: 77-85
3. Canavero S, Bonicalzi V, editors. Central pain syndrome. Cambridge: Cambridge University Press; 2011. p.
4. Canavero S. HEAVEN: The head anastomosis venture Project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int. 2013. 4: S335-42
5. Canavero S, Ren X, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI). Surgery. 2016. 160: 11-9
6. Canavero S, Ren X. Houston, GEMINI has landed: Spinal cord fusion achieved. Surg Neurol Int. 2016. 7: S626-8
7. Canavero S, Ren X, editors. The technology of head transplantation. New York: NOVA Science; 2020. p.
8. Canavero S, Ren X, Kim CY. Heterologous spinal cord transplantation in man. Surg Neurol Int. 2021. 12: 295
9. Frost CSalous AKetheeswaran SNgaage LMHanwright PJGhergherehchi C. Polyethylene glycol fusion restores axonal continuity and improves return of function in a rat median nerve denervation model. https://pubmed.ncbi.nlm.nih.gov/37734115/.
10. Kim CY, Oh H, Ren X, Canavero S. Immunohistochemical evidence of axonal regrowth across polyethylene glycol-fused cervical cords in mice. Neural Regen Res. 2017. 12: 149-50
11. Kouhzaei S, Rad I, Khodayari K, Mobasheri H. The neuroprotective ability of polyethylene glycol is affected by temperature in ex vivo spinal cord injury model. J Membr Biol. 2013. 246: 613-9
12. Laverty PH, Leskovar A, Breur GJ, Coates JR, Bergman RL, Widmer WR. A preliminary study of intravenous surfactants in paraplegic dogs: Polymer therapy in canine clinical SCI. J Neurotrauma. 2004. 21: 1767-77
13. Liu C, Huang Y, Pang M, Yang Y, Li S, Liu L. Tissue-engineered regeneration of completely transected spinal cord using induced neural stem cells and gelatin-electrospun poly (lactide-co-glycolide)/polyethylene glycol scaffolds. PLoS One. 2015. 10: e0117709
14. Marzullo TC, Britt JM, Stavisky RC, Bittner GD. Cooling enhances in vitro survival and fusion-repair of severed axons taken from the peripheral and central nervous systems of rats. Neurosci Lett. 2002. 327: 9-12
15. Mohrman AE, Farrag M, Grimm RK, Leipzig ND. Evaluation of in situ gelling chitosan-PEG copolymer for use in the spinal cord. J Biomater Appl. 2018. 33: 435-46
16. Olby NJ, Muguet-Chanoit AC, Lim JH, Davidian M, Mariani CL, Freeman AC. A placebo-controlled, prospective, randomized clinical trial of polyethylene glycol and methylprednisolone sodium succinate in dogs with intervertebral disk herniation. J Vet Intern Med. 2016. 30: 206-14
17. Ren S, Liu Z, Kim CY, Fu K, Wu Q, Hou L. Reconstruction of the spinal cord of spinal transected dogs with polyethylene glycol. Surg Neurol Int. 2019. 10: 50
18. Ren X, Kim CY, Canavero S. Bridging the gap: Spinal cord fusion as a treatment of chronic spinal cord injury. Surg Neurol Int. 2019. 10: 51
19. Ryan I, Henderson PW, Canavero S, Ren X, editors. GEMINI: Overview of fusogens. The technology of head transplantation. New York: NOVA Science; 2020. p. 51-68
20. Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021. 51: 861-3
21. Yari-Ilkhchi A, Ebrahimi-Kalan A, Farhoudi M, Mahkam M. Design of graphenic nanocomposites containing chitosan and polyethylene glycol for spinal cord injury improvement. RSC Adv. 2021. 11: 19992-20002
22. Yang Y, Zhang L, Huang M, Sui R, Khan S. Reconstruction of the cervical spinal cord based on motor function restoration and mitigation of oxidative stress and inflammation through eNOS/Nfr2 signaling pathways using ibuprofen-loaded nanomicelles. Arab J Chem. 2021. 14: 103289
23. Zhang C, Wang A, Zhang G, Wu C, Rong W, Huo X. Experimental study of electric field stimulation combined with polyethylene glycol in the treatment of spinal cord injury in rats. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2022. 39: 10-8
24. Zhang C, Wang A, Zhang G, Rong W, Wu C, Huo X. Effects of the combination therapy of electric field stimulation and polyethylene glycol in the ex vivo spinal cord of female rats after compression. J Neurosci Res. 2021. 99: 1850-63