- Department of Neurosurgery, School of Medicine, Loma Linda University, Loma Linda, California, United States.
- Department of Neurosurgery, Loma Linda University School of Medicine, Loma Linda, California, United States.
Correspondence Address:
Miguel Angel Lopez-Gonzalez, Department of Neurosurgery, Loma Linda University School of Medicine, Loma Linda, California, United States.
DOI:10.25259/SNI_60_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brandon Michael Edelbach1, Miguel Angel Lopez-Gonzalez2. Percutaneous high cervical spinal cord stimulation for refractory trigeminal neuralgia. 08-Jun-2023;14:198
How to cite this URL: Brandon Michael Edelbach1, Miguel Angel Lopez-Gonzalez2. Percutaneous high cervical spinal cord stimulation for refractory trigeminal neuralgia. 08-Jun-2023;14:198. Available from: https://surgicalneurologyint.com/surgicalint-articles/12357/
Abstract
Background: Trigeminal neuralgia (TN) is a debilitating pain that affects the dermatomes associated with the trigeminal nerve (V1, V2, and V3). Unfortunately, many medical treatments and surgical procedures fail to sufficiently modulate the pain associated with this condition.
Case Description: This study presents two extreme cases of refractory TN (RTN) that progressed to atypical facial pain and describes successful mitigation of the neuralgia of said cases by percutaneous implantation of upper cervical spinal cord stimulation (SCS). The SCS was designed to target the descending spinal trigeminal tract.
Conclusion: Together, these cases collaborate with the limited literature and further delineate the use and potential advantages of SCS in the treatment of RTN.
Keywords: Descending spinal trigeminal tract, Facial pain, Neuromodulation, Spinal cord stimulation, Trigeminal neuralgia
INTRODUCTION
Trigeminal neuralgia (TN) is a syndrome distinguished by unilateral, paroxysmal, and electric pain that is most often found in the V2 and V3 dermatomes of the cranial nerve five (CN V).[
The first line of treatment for TN is pharmacological, with the most common treatment methods being carbamazepine or oxcarbazepine.[
A line of therapy developing for RTN is based on neuromodulation. At present, there are few clinically reported methods of neuromodulation: Motor cortex stimulation,[
The selection criteria for the following patients was a RTN that progressed to trigeminal neuropathic pain, as a result of injury to trigeminal nerve by prior procedures, and described as unremitting throbbing and burning pain in the affected areas.[
CASE PRESENTATION
Case 1
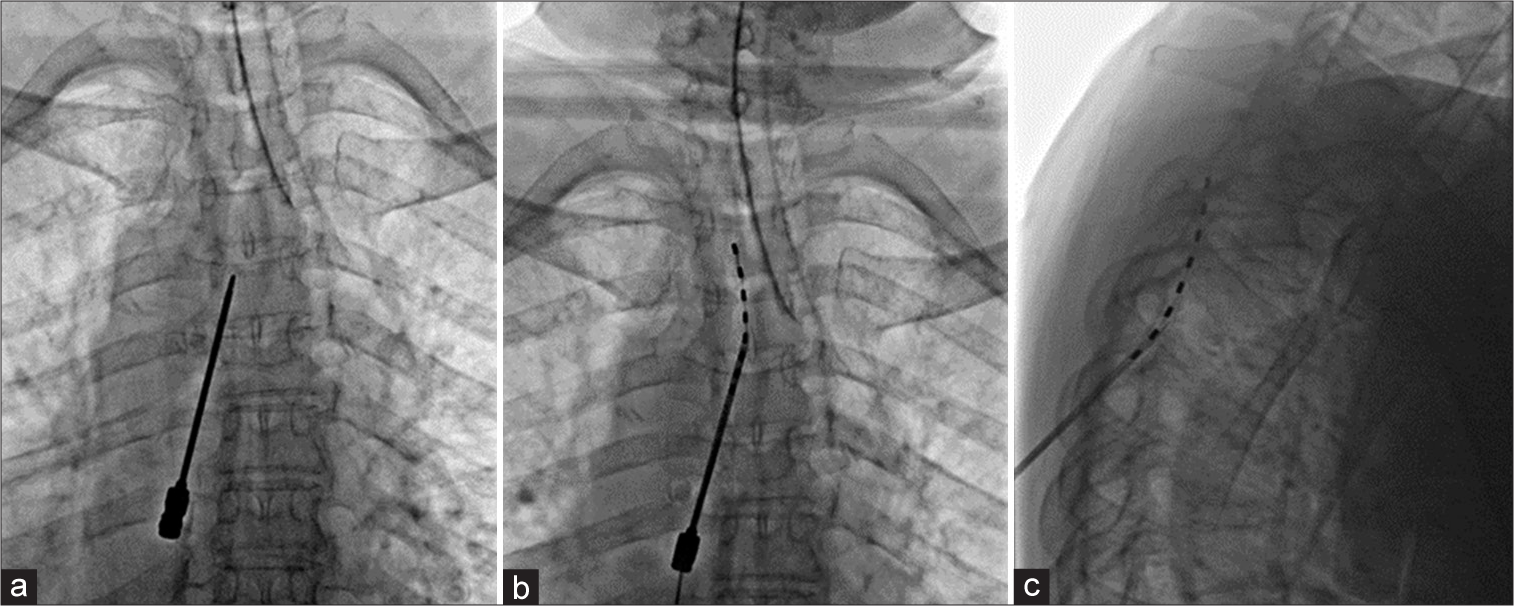
A 76-year-old woman presented with RTN. The patient had a history of the right TN at the V1 and V2 distribution after a neurofibroma was resected from the pterygopalatine fossa at another institution. This was followed by a right balloon compression of the gasserian ganglion. Subsequently, the patient reported no improvement in pain. This was followed by a right retrosigmoid craniectomy for microvascular decompression, which also did not improve her pain. Retrosigmoid craniectomy was followed by a resection of V2 surgical scar soft-tissue mass after which the patient reported still no improvement. The patient also had a history of Botox injections that reduced pain for 2–3 months, as well as several nerve blocks that gave the patient only a temporary reprieve. She was followed by pain management physicians and headache neurologists with no improvement in pain (oxycodone, methadone, and lidocaine gel). The patient was later referred to our evaluation with a constant burning and paroxysmal stabbing right facial pain accompanied by nausea affecting the distal portion of V2 and V3. This pain was aggravated by talking, chewing, brushing teeth, smiling, and swallowing. The patient reported that pain prevented adequate sleep and social function with a constant severity of 10/10 on a visual analog scale (VAS). Due to the failure of these surgical and medical procedures to provide pain relief, the patient was recommended for a percutaneous trial of SCS with the intention of targeting the descending spinal trigeminal tract. The patient underwent a neuropsychological evaluation and was deemed an appropriate candidate for the trial (ruled out unresolved major psychiatry comorbidities, drug abuse, and unresolved secondary gain). She also had brain, cervical, and thoracic Magnetic resonance imaging (MRI) preoperatively. Under general anesthesia, the patient was placed in the prone position and fluoroscopy was used to identify the level of the thoracic entry point on the skin (T6) of the Tuohy needle and in the epidural space in T4 [
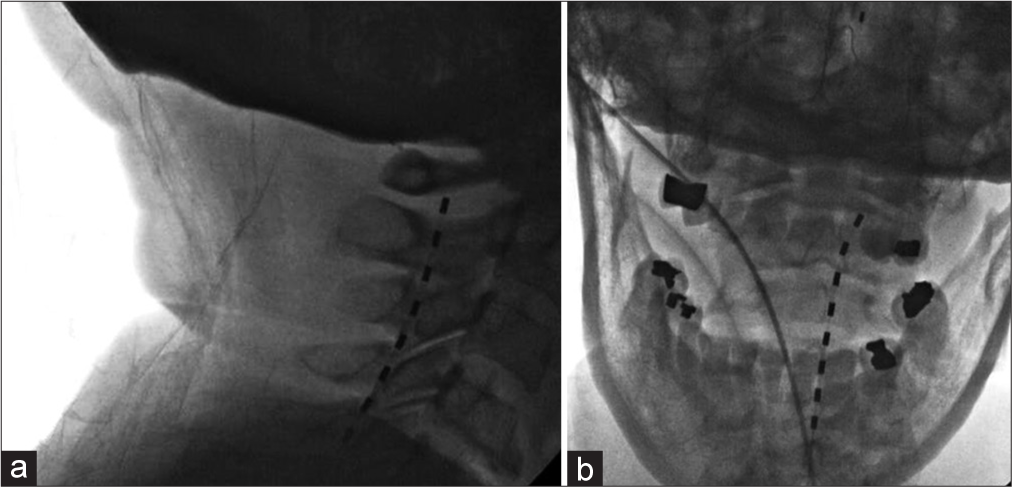
Then, a percutaneous lead of eight contacts (Medtronic 977A275 Lead Restore SureScan 75 mm compact 1 × 8) was advanced along the posterior epidural thoracic and cervical space under the guidance of fluoroscopy until the level of C1–C2 on the right side, at which point the lead was then secured in place. The needle was then removed and the lead was fixed in place using tape secured to the patient’s skin. Then, X-rays were taken to determine the level of placement if the trial surgery was successful [
Figure 2:
Lateral fluoroscopy view after the placement of the spinal cord stimulation (SCS) trial electrode (dashed line image), advanced to the upper cervical spine just below posterior arc of C1 level in the dorsal epidural space (a); Anterior-posterior fluoroscopy view showing the electrode (dashed line image) towards right side of the epidural space in the upper cervical spine (b).
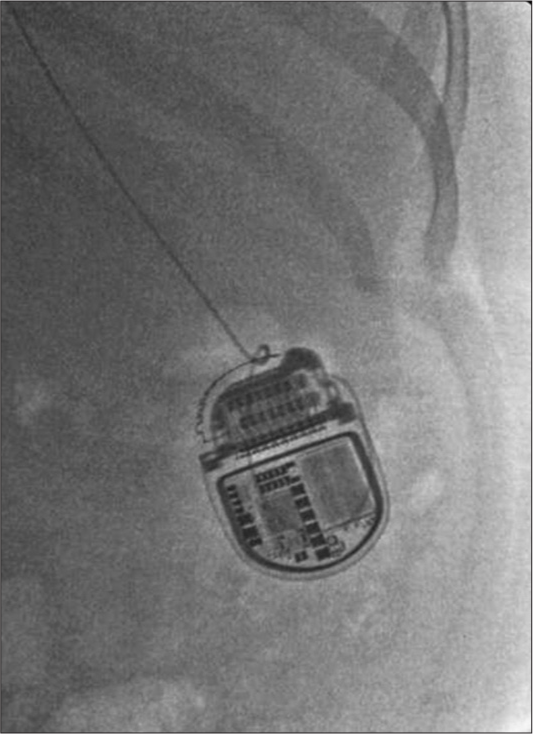
The patient was kept in a soft collar to avoid neck rotation and migration of stimulation, and the temporary lead was uneventfully removed 7 days later in the outpatient clinic. The patient reported that during the high-frequency stimulation period, she had a 60% reduction in pain. As such, the patient was then considered for the placement of a permanent SCS implant. The patient agreed to the permanent placement of the lead and as such was admitted back to the operating room. The patient was prepared for surgery and again with a similar technique to that of the trial implantation, replicating the placement of the cervical lead, except that she now had a lead anchored to the fascia in the thoracic area (requiring a 4 cm incision) and this was tunneled to the right posterior flank for the implantation of the subcutaneous battery (Medtronic 97715 Stim Medt Nerve Intellis). The incisions were then closed and properly covered and the patient recovered in stable conditions. Overall, with a short follow-up of 4 weeks (using two groups of stimulation, with 300 Hz, pulse width 170 ms, and varying intensities between 0.5 and 0.8 mA), the patient referred a significant improvement in her quality of life since her first stimulation session with 40% pain relief and referring to eating and talking more comfortably and rating her pain 6/10. No wound complications were encountered.
Case 2
A 29-year-old woman had severe pain since 2006 on the right V1 and V2 distribution of the trigeminal nerve was referred to our clinic. The patient described this pain as initially a lacerating, electric, and shooting pain that lasted a few seconds to several minutes. She reported that this pain was triggered by wind, brushing teeth, stress, eating, cleaning her face, and bright light. The patient received several medical treatments including lamotrigine, phenytoin, gabapentin, topiramate, carbamazepine, clonazepam, celecoxib, nortriptyline, baclofen, hydroxyzine, sumatriptan, and rimegepant, all of which were ineffective in treating pain.
She was then treated with microvascular decompression in 2017 which was effective at treating her pain for 2 years. Subsequent recurrence of pain along the V1 and V2 dermatomes brought the patient back for further treatment. The patient’s RTN was treated with Gamma Knife surgery which had minimal effects on her pain and later with balloon compression, also unsuccessful. Her pain then became atypical with constant stabbing and burning sensation and allodynia. At this point, the patient reported that the pain was constant and interrupted her sleep with VAS 10/10 severity of the pain and was referred for our evaluation. She underwent neuropsychological evaluation, and brain, cervical, and thoracic magnetic resonance imaging, and was considered a candidate for the percutaneous upper cervical stimulation trial.
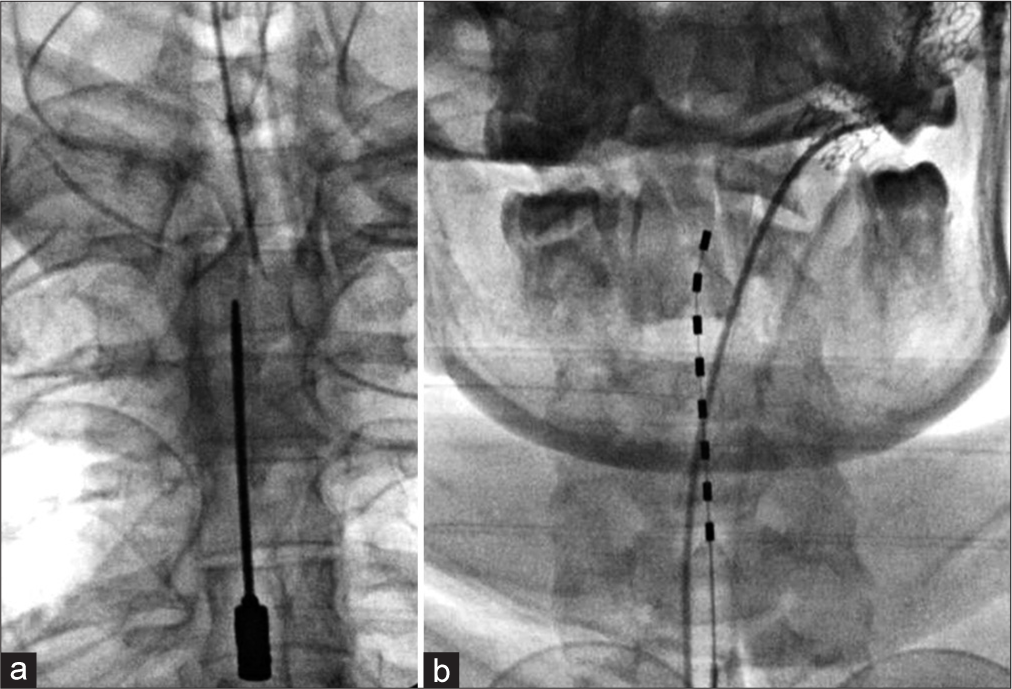
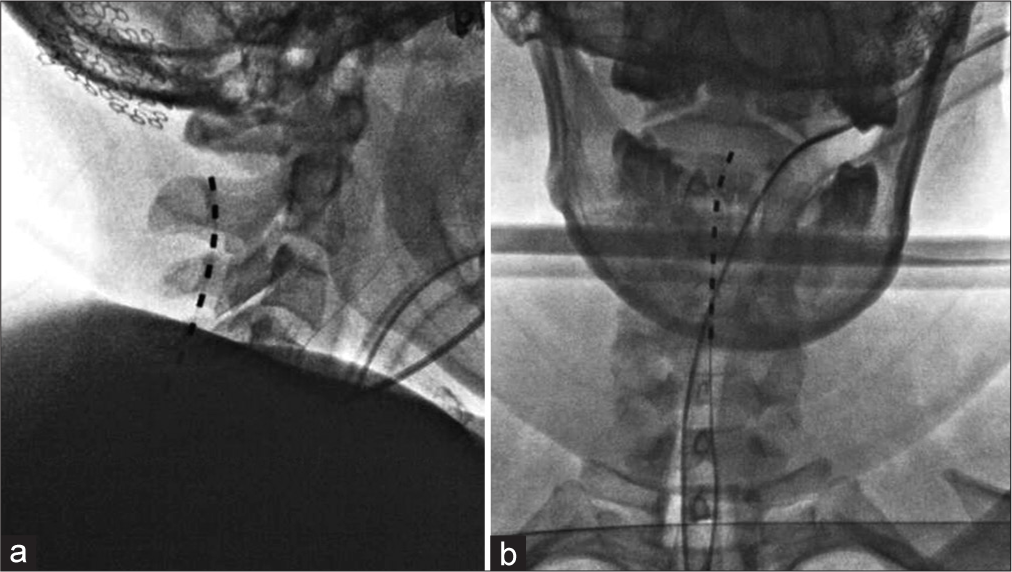
The patient was then prepared for the trial surgery in the prone position and under general anesthesia. With fluoroscopy guidance, a Tuohy needle was inserted into the epidural space at the T3–T4 level. From this point on, the percutaneous SCS lead advanced to the level of C2–C4 that has resistance to further advancement [
The patient reported a 90% pain relief with high-frequency stimulation (rate 300 Hz, pulse width 170 ms, and varying intensity from 1.8 to 2.2 mA) with a significant improvement in quality of life. With the success of the trial procedure, the patient was then readmitted to the OR replicating the initial trial procedure [
At 6 months after surgery, the patient reported between 60% and 98% reduction in both severity of pain and reduction in frequency of attacks (2-3 times per week), rather than lasting about 10 days. The patient was consequentially able to resume more normal daily activities and take significantly less pain medications.
DISCUSSION
The techniques applied in each of the presented cases utilize high-frequency stimulation of the upper cervical spinal cord with the intention of targeting the descending trigeminal spinal nucleus that receives input from V3 branch. This anatomical distribution does not cover all the anatomical basis for ophthalmic nerve (V1) and mandibular nerve (V2) pain, although is able to offer partial coverage on the pain distribution. These two case examples are extreme cases of RTN with subsequent transformation to atypical facial pain after multiple invasive procedures. It is important to select appropriate patient candidates with realistic goals of treatment. Formal neuropsychological testing is required to identify problematic emotional reactions, maladaptive thinking and behavior, and social issues that can contribute to pain and disability that can affect the surgical outcomes.[
Additional evidence presented by Velasquez et al. suggested that the dorsal horn islet may convey the therapeutic effects of SCS.[
Only eight other case reports[
CONCLUSION
Our study supports the use of SCS as a viable and safe treatment option for individuals with RTN, and highlights the necessity of conducting randomized controlled trials on the treatment of RTN through neuromodulation of the descending trigeminal spinal nucleus.
Statement of ethics
Ethical approval and patient’s consent is not required for retrospective case report studies without identifiable information in accordance with Loma Linda University Institutional Review Board guidelines.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Barolat G, Knobler RL, Lublin FD. Trigeminal neuralgia in a patient with multiple sclerosis treated with high cervical spinal cord stimulation. Case report. Appl Neurophysiol. 1988. 51: 333-7
2. Campbell CM, Jamison RN, Edwards RR. Psychological screening/phenotyping as predictors for spinal cord stimulation. Curr Pain Headache Rep. 2013. 17: 307
3. Chen F, Niu Y, Meng F, Xu P, Zhang C, Xue Y. Recurrence rates after microvascular decompression in patients with primary trigeminal neuralgia and its influencing factors: A systematic review and meta-analysis based on 8,172 surgery patients. Front Neurol. 2021. 12: 738032
4. Chung M, Huh R. Neuromodulation for trigeminal neuralgia. J Korean Neurosurg Soc. 2022. 65: 640-51
5. Cruccu G, Finnerup NB, Jensen TS, Scholz J, Sindou M, Svensson P. Trigeminal neuralgia. Neurology. 2016. 87: 220-8
6. Cruccu G, Gronseth G, Alksne J, Argoff C, Brainin M, Burchiel K. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008. 15: 1013-28
7. Dieleman JP, Kerklaan J, Huygen FJ, Bouma PA, Sturkenboom MC. Incidence rates and treatment of neuropathic pain conditions in the general population☆. Pain. 2008. 137: 681-8
8. Ebel H, Rust D, Tronnier V, Böker D, Kunze S. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir (Wien). 1996. 138: 1300-6
9. Eller JL, Raslan AM, Burchiel KJ. Trigeminal neuralgia: Definition and classification. Neurosurg Focus. 2005. 18: E3
10. Floridia D, Cerra F, Corallo FP, Di Cara M, Spartà S, Nania G. Efectiveness of high-frequency cervical spinal cord stimulation in the treatment of refractory trigeminal neuropathy. Medicine (Baltimore). 2020. 99: e22304
11. Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K. Practice parameter: The diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008. 71: 1183-90
12. Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years. Eur J Pain. 2019. 23: 652-9
13. Joffroy A, Levivier M, Massager N. Trigeminal neuralgia. Pathophysiology and treatment. Acta Neurol Belg. 2001. 101: 20-5
14. Jones MR, Baskaran AB, Rosenow JM. Cervical spinal cord stimulation for facial pain. Prog Neurol Surg. 2020. 35: 133-40
15. Jones MR, Rosenow JM. Importance of laterality in cervical spinal cord stimulation for facial pain: Case report and anatomic review. Oper Neurosurg (Hagerstown). 2020. 19: E83-6
16. Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. p. 482-6
17. Karam SD, Tai A, Snider JW, Bhatia S, Bedrick EJ, Rashid A. Refractory trigeminal neuralgia treatment outcomes following CyberKnife radiosurgery. Radiat Oncol. 2014. 9: 257
18. Kumar K, Toth C, Nath RK. Deep brain stimulation for intractable pain: A 15-year experience. Neurosurgery. 1997. 40: 736-46
19. Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia-a prospective systematic study of clinical characteristics in 158 patients. Headache. 2014. 54: 1574-82
20. McQuay H, Carroll D, Jadad AR, Wiffen P, Moore A. Anticonvulsant drugs for management of pain: A systematic review. BMJ. 1995. 311: 1047-52
21. Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015. 11: 289-99
22. Papa A, Di Dato MT, Buonavolontà P, Tammaro D, Pironti A, Saracco EE. Craniofacial pain and high frequency stimulation. Pathos. 2016. 23: 2
23. Peirs C, Dallel R, Todd AJ. Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia. J Neural Transm (Vienna). 2020. 127: 505-25
24. Peter Chang BA, Levy RM. High lateral cervical spinal cord stimulation (SCS) for neuropathic facial pain: Report of 10 cases: 943. Neurosurgery. 2010. 67: 550
25. Rajashekar D, Tambirajoo K, Franceschini P, Eldridge P. Longterm outcomes of high cervical spinal cord stimulation in the management of neuropathic facial pain. Int J Surg. 2017. 47: S10
26. Texakalidis P, Tora MS, McMahon JT, Greven A, Anthony CL, Nagarajan P. Percutaneous trigeminal stimulation for intractable facial pain: A case series. Neurosurgery. 2020. 87: 547-54
27. Velásquez C, Tambirajoo K, Franceschini P, Eldridge PR, Farah JO. Upper cervical spinal cord stimulation as an alternative treatment in trigeminal neuropathy. World Neurosurg. 2018. 114: e641-6
28. Zhao L, Song T. Case report: Short-term spinal cord stimulation and peripheral nerve stimulation for the treatment of trigeminal postherpetic neuralgia in elderly patients. Front Neurol. 2021. 12: 713366