- Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq
- Department of Surgery, University of Baghdad, College of Medicine, Baghdad, Iraq
- Department of Surgery, Al-Nahrain University, College of Medicine, Baghdad, Iraq
- Department of Family Medicine, Faculty of Medicine and Pharmacy, Mohammed I University, Oujda, Morocco
- Department of Surgery, Medical College of Georgia, Augusta, Georgia
- Department of Surgery, Dow University of Health Sciences, Karachi, Pakistan
- Department of Neurosurgery, University of Cincinnati, United States
- Department of Neurosurgery, University of Pittsburgh, Pittsburgh, United States
Correspondence Address:
Mustafa Ismail, Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq.
DOI:10.25259/SNI_427_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mustafa Ismail1, Ali Al-Shalchy2, Younus M. Al-Khazaali3, Abdelilah Lahmar4, Liam V. Goldman5, Mostafa H. Algabri2, Danisha Kumar6, Paolo Palmisciano7, Samer S. Hoz8. Perioperative blood transfusion management in surgical resection of intracranial meningiomas: A meta-analysis. 26-Jul-2024;15:256
How to cite this URL: Mustafa Ismail1, Ali Al-Shalchy2, Younus M. Al-Khazaali3, Abdelilah Lahmar4, Liam V. Goldman5, Mostafa H. Algabri2, Danisha Kumar6, Paolo Palmisciano7, Samer S. Hoz8. Perioperative blood transfusion management in surgical resection of intracranial meningiomas: A meta-analysis. 26-Jul-2024;15:256. Available from: https://surgicalneurologyint.com/surgicalint-articles/13014/
Abstract
Background: Gross total resection (GTR) of intracranial meningiomas is curative in most cases. However, perioperative blood transfusions may be necessary for complex skull bases and/or high-grade meningiomas. Guidelines for blood transfusions during intracranial meningioma surgery remain unclear. This scoping review aims to delineate the main characteristics of patients who underwent intracranial meningioma surgery, the prevalence of the selected patients who required blood transfusions, and common causes for transfusion.
Methods: A scoping review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Extension for Scoping Reviews guidelines to include studies reporting eligibility, protocols, and potential complications related to blood transfusion within the perioperative management of intracranial meningiomas.
Results: A total of 33 articles encompassing 3009 meningioma patients were included in the study. The most common symptom was headache (18%), and the most frequent type of meningioma was World Health Organization grade-1 meningothelial (50.4%). The lateral supraorbital approach was the most common surgical corridor (59.1%) in skull base meningiomas, and most patients underwent GTR (69%). Blood transfusion was required for 20% of patients, with a mean estimated intraoperative blood loss of 703 mL (ranging from 200 mL to 2000 mL). The main indications for blood transfusion in meningioma surgery were intraoperative blood loss (86%) and preoperative anemia (7.3%).
Conclusion: This scoping found that 20% of the included patients required blood transfusion. It also points out that several factors could influence the necessity for a transfusion, encompassing surgical blood loss, pre-existing anemia, and the surgery’s length. This scoping review may provide surgeons with a potential guide to inform their decision-making process regarding blood transfusions during meningioma surgeries.
Keywords: Blood loss, Blood transfusion, Intracranial meningioma, Surgical resection
INTRODUCTION
Meningiomas represent the most common type of brain tumor, accounting for more than 35% of all brain tumors according to the Central Brain Tumor Registry of the United States between 2006 and 2010.[
Numerous neurosurgery procedures may be associated with significant blood loss, having the neurosurgical team ready to plan and promptly request blood products intraoperatively. Most research is focused on transfusions in subarachnoid hemorrhage, traumatic brain injury, and spine surgery.[
In part due to the scarcity of relevant literature, blood transfusion guidelines after intracranial meningioma surgery have not been developed, with the current neurosurgical literature lacking any decisive knowledge on the topic. In this scoping review, we analyzed the literature to elucidate the selected patients’ characteristics, the prevalence of patients who required blood transfusion among intracranial meningioma surgery, and the common causes of such transfusions.
MATERIALS AND METHODS
Literature search
A scoping review of the literature was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses-Extension for Scoping Reviews guidelines.[
Study selection
The inclusion and exclusion criteria for the study were outlined a priori. Studies were included if they: (1) included ≥1 patient with histologically-proven intracranial meningioma; (2) reported available data on preoperative embolization and/or surgical management of underlying tumor, along with clinical characteristics and outcomes of intracranial meningioma; (3) mentioned the use of blood transfusion perioperatively (i.e., intra-operative or during postoperative hospitalization); and (4) were written in English. Studies were excluded if they: (1) were book chapters, conference abstracts, reviews, animal or cadaver studies; (2) featured patients with intracranial meningioma with no record of perioperative blood transfusion; (3) lacked a clear differentiation between patients with intracranial meningioma and those without; and (4) lacked adequate clinical data on intracranial meningioma management and/or blood transfusion.
Two reviewers (A.L. and L.G.) independently examined the titles and abstracts of the gathered publications and then evaluated the full texts of the studies that met the inclusion criteria. A third reviewer (M.I.) arbitrated any differences of opinion. According to the predetermined inclusion criteria, eligible publications were included, and references were explored for additional relevant studies.
Data extraction
One reviewer (A.L.) extracted the data, which was then validated by two independent reviewers (L.G. and M.I.). The following data were extracted: authors, year of publication, age, gender, clinical presentation, tumor location, histologic grade and type based on the WHO guidelines of central nervous system tumors available at the time of publication, molecular features, surgical approach, and duration, extent of resection, intraoperative estimated blood loss, extent and time of blood transfusion (i.e., intraoperatively and postoperatively), reoperation/other procedures, complications, and survival status. For articles with available data, the extent of tumor resection was categorized based on the Simpson grading[
Data synthesis, quality assessment, and statistical analysis
The primary outcomes of interest were the characteristics and eligibility for blood transfusion of the included patients undergoing surgical resection of intracranial meningiomas. The secondary outcomes of interest were the additional factors related to blood loss and transfusion, including the amount of blood loss and the average blood transfusion quantity. The risk of bias was assessed by two independent authors (M.I. and A.L.) using the JBI checklists.[
RESULTS
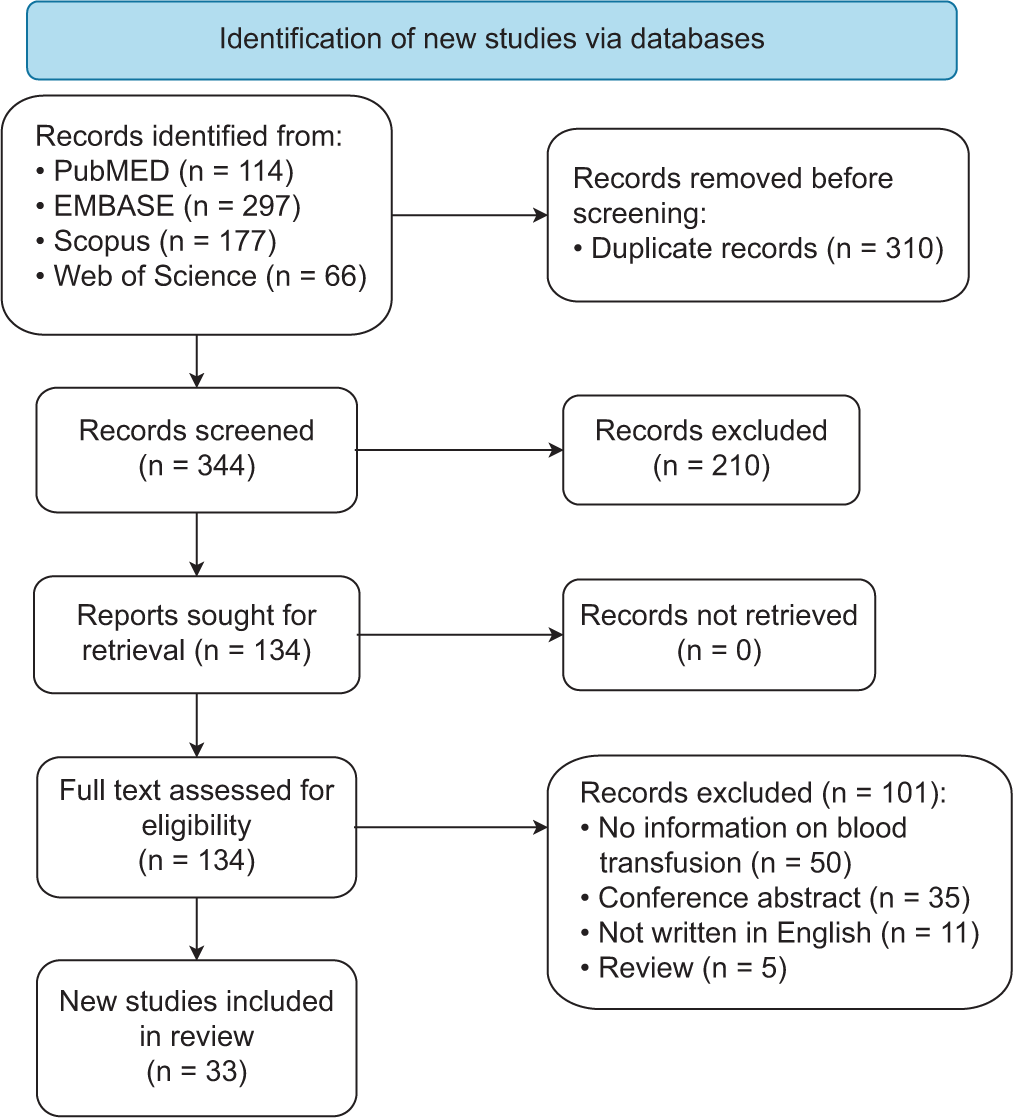
Study selection
Demographics and clinicopathological features
A total of 3009 patients were reviewed. The mean age at tumor diagnosis was 51 years (range 14–87), with a female prevalence (71.4%) [
Management strategies for tumor resection
The most common surgical corridors for tumor resection across papers that discussed surgical approaches for skull base lesions were the lateral supraorbital (59.1%), frontotemporal (30.9%), and Kawase (9.2%) approaches. The mean duration of the surgeries was 5.2 h (ranging from 1.5 to 14.3 h). Data on the extent of the tumor resection were available for 342 cases.[
Blood loss and transfusion management
The mean estimated intraoperative blood loss was 703 mL (1.41 units, ranging from 200 mL to 2000 mL). A total of 609 patients (20%) needed a blood transfusion, and the mean intraoperative estimated blood transfusion was 579 mL (1.15 blood units) (Ranging from 0.39 to 87 blood units). In addition, we found that the most common cause for transfusion was blood loss during surgery (86%), followed by preoperative anemia (7.3%). A total of 18 patients received postoperative blood, and 11 cases required 14 units of blood while they were hospitalized. Four hundred and sixty-two patients underwent preoperative embolization of intracranial meningioma.
Postoperative complications and other outcomes
Additional treatments were required in 47 cases: 26 patients (55%) necessitated repeated surgical exploration for suspected recurrence, and 17 (36%) were re-operated for the development of brain herniation. O’Reilly and Hamilton reported that one patient was diagnosed with intracerebral hemorrhage that necessitated reopening.[
DISCUSSION
The neurosurgical literature frequently discusses the role of blood transfusion in cerebrovascular, trauma, and spine but scarcely reports its benefits and nuances in neurooncology.[
Several measures for blood conservation have been implemented in an effort to reduce homologous blood transfusion. Messmer and Sunder-Plassman established a multitude of blood conservation procedures, including autologous blood extraction.[
In our review of 3009 resected meningiomas, we found that patients lost a mean of 703 mL, and 609 patients were transfused with a mean of 579 mL of blood. Neef et al. found that 16.1% of the 423 patients surgically treated for meningioma resection received RBC transfusions, noting that these patients were significantly older (P = 0.0153) and more likely to have comorbidities such as cardiovascular disease (P = 0.0107) and diabetes (P = 0.0210). The authors also found that tumor size in the RBC transfusion group was significantly larger (P < 0.0001) compared to the nonRBC transfusion group.[
The different locations of intracranial meningiomas may also affect the decision-making of ordering blood transfusion perioperatively. Blood transfusion during convexity meningioma surgery is a typical procedure used to restore blood loss and maintain proper blood pressure and oxygenation of the patient. The decision to perform a transfusion is primarily influenced by various possible factors which include the patient’s age, pre-operative hemoglobin levels, the length of surgery, and the surgeon’s experience and preferences. Depending on the patient’s medical history and the surgeon’s decision, autologous or allogenic blood may be used for blood transfusions during this type of surgery. Blood transfusions pose risks such as transfusion reactions, the transmission of infections, and an increased chance of bleeding.[
Preoperative embolization of intracranial meningioma may have several advantages. A total of 462 patients across our pooled studies have received preoperative embolization anteceding meningioma resection. Several primary benefits of preoperative embolization for meningioma surgery have been outlined.[
Limitations
Several limitations of this review should be mentioned. The lack of individual-patient data prevented to conduct of additional statistical analyses, such as correlations and analyses, to identify the risks of extensive blood loss during intracranial meningioma surgery based on multiple parameters, including the meningioma size, location, and grading and how this may impact the need of blood transfusion. Six case reports were included, adding a lower level of evidence. There is limited data on the various blood product transfusions during meningioma surgery, and the literature also lacks data on the complications that could arise from them. Different anesthesia schemes were not reported in detail across the included studies. However, this is the first review that summarizes the current literature on the topic, advocating the need to conduct multicenter prospective databases to develop standardized guidelines for planning perioperative blood transfusion schemes during intracranial meningioma resection.
CONCLUSION
This scoping found 20% of the patients that underwent intracranial meningioma surgery, which required blood transfusion. Our review also pointed out that, during meningioma surgery, the need for a blood transfusion can be impacted by a number of variables, including blood loss due to the surgery, preoperative anemia, and the duration of the surgery. This scoping review may give the surgeons a possible map to aid in the decision of blood transfusion during intracranial meningioma surgery.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY FILE
Supplementary File 1. Risk of bias assessments for included studies
Joanna Briggs Institute Checklist for Case Reports – Criteria
Were patient’s demographic characteristics clearly described? Was the patient’s history clearly described and presented as a timeline? Was the current clinical condition of the patient on presentation clearly described? Were diagnostic tests or assessment methods and the results clearly described? Was the intervention (s) or treatment procedure (s) clearly described? Was the post-intervention clinical condition clearly described? Were adverse events (harms) or unanticipated events identified and described? Does the case report provide takeaway lessons?
Responses Options: Yes, No, Unclear, Not Applicable (NA)
Quality Rating: Poor 0 – 2; Fair 3 – 5; Good 6 – 8
Joanna Briggs Institute Checklist for Case Series – Criteria
Were there clear criteria for inclusion in the case series? Was the condition measured in a standard, reliable way for all participants included in the case series? Were valid methods used for identification of the condition for all participants included in the case series? Did the case series have consecutive inclusion of participants? Did the case series have complete inclusion of participants? Was there clear reporting of the demographics of the participants in the study? Was there clear reporting of clinical information of the participants? Were the outcomes or follow up results of cases clearly reported? Was there clear reporting of the presenting site (s)/clinic (s) demographic information? Was statistical analysis appropriate?
Responses Options: Yes, No, Unclear, Not Applicable (NA)
Quality Rating: Poor 0 – 3; Fair 4 – 7; Good 8 – 10
References
1. Alan N, Seicean A, Seicean S, Neuhauser D, Weil RJ. Impact of preoperative anemia on outcomes in patients undergoing elective cranial surgery. J Neurosurg. 2014. 120: 764-72
2. Ali Z, Khan T, Jangra K, Ubaid S, Bashir H, Wani AA. An evaluation of the practice for the requisition of blood products and its utilization in neurosurgical patients undergoing elective surgery at a tertiary care hospital. Anesth Essays Res. 2020. 14: 160-4
3. Bagwe S, Chung LK, Lagman C, Voth BL, Barnette NE, Elhajjmoussa L. Blood transfusion indications in neurosurgical patients: A systematic review. Clin Neurol Neurosurg. 2017. 155: 83-9
4. Baily DN, Bare JR. Chemical and hematological changes in stored blood. Transfus Paris. 1975. 17: 224
5. Barrie U, Youssef CA, Pernik MN, Adeyemo E, Elguindy M, Johnson ZD. Transfusion guidelines in adult spine surgery: A systematic review and critical summary of currently available evidence. Spine J. 2022. 22: 238-48
6. Bendszus M, Klein R, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. Am J Neuroradiol. 2000. 21: 255-61
7. Black PM, Morokoff AP, Zauberman J. Surgery for extra-axial tumors of the cerebral convexity and midline. Neurosurgery. 2008. 62: 1115-23
8. Catapano JS, Whiting AC, Mezher AW, Przybylowski CJ, See AP, Labib MA. Postembolization change in magnetic resonance imaging contrast enhancement of meningiomas is a better predictor of intraoperative blood loss than angiography. World Neurosurg. 2020. 135: e679-85
9. Chand S, Mani A, Amita R, Gupta D. Patient blood management in a neurosurgical patient with anti-e antibody. Asian J Transfus Sci. 2021. 15: 226-8
10. Dean BL, Flom RA, Wallace R, Khayata MH, Obuchowski NA, Hodak JA. Efficacy of endovascular treatment of meningiomas: Evaluation with matched samples. AJNR Am J Neuroradiol. 1994. 15: 1675-80
11. Doran SE, Henry TR, Bockenstedt PL, Ross DA. Uncomplicated stereotactic and open neurosurgical procedures in patients with factor VII deficiency. Surg Neurol. 1994. 42: 79-82
12. Dross P. Preoperative transcatheter embolization of an unusual intraorbital and intracranial meningioma. J Tenn Med Assoc. 1987. 80: 674-6
13. Firth PG, Szabo MD. Autologous transfusion of a neurosurgical patient with sickle cell disease. J Neurosurg Anesthesiol. 2010. 22: 180
14. Hegazy A, Al-Shami H, Ali MF, Fathallah M, Salah A, Mohamed H. Mobilization of the outer cavernous membrane decreases bleeding and improves resection in spheno-clinoidal meningiomas without cavernous sinus extension: A randomized controlled trial. Neurol India. 2018. 66: 407-15
15. Hieshima G, Everhart F, Mehringer C, Tsai F, Hasso AH, Grinnell VS. Preoperative embolization of meningiomas. Surg Neurol. 1980. 14: 119-27
16. Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017. 41: 132-8
17. Howick JChalmers IGlasziou PGreenhalgh THeneghan CLiberati A. Oxford centre for evidence-based medicine 2011 levels of evidence. Available from: https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf [Last accessed on 2024 May 27].
18. Kai Y, Hamada JI, Morioka M, Yano S, Nakamura H, Makino K. Preoperative cellulose porous beads for therapeutic embolization of meningioma: Provocation test and technical considerations. Neuroradiology. 2007. 49: 437-43
19. Karadimov D, Binev K, Nachkov Y, Platikanov V. Use of activated recombinant Factor VII (NovoSeven) during neurosurgery. J Neurosurg Anesthesiol. 2003. 15: 330-2
20. Lagman C, Ong V, Nguyen T, Alkhalid Y, Sheppard JP, Romiyo P. The Meningioma Vascularity Index: A volumetric analysis of flow voids to predict intraoperative blood loss in nonembolized meningiomas. J Neurosurg. 2018. 130: 1547-52
21. Lagman C, Sheppard JP, Beckett JS, Tucker AM, Nagasawa DT, Prashant GN. Red blood cell transfusions following resection of skull base meningiomas: Risk factors and clinical outcomes. J Neurol Surg B Skull Base. 2018. 79: 599-605
22. Lee SS, Chan KY, Pang KH, Datta N, Poon YF, Aung TH. Effect of preoperative embolization on resection of intracranial meningioma: Local experience. Surg Pract. 2006. 10: 106-10
23. Manelfe C, Lasjaunias P, Ruscalleda J. Preoperative embolization of intracranial meningiomas. Am J Neuroradiol. 1986. 7: 963-72
24. Messmer K. Circulatory significance of hemodilution: Rheological changes and limitations. Adv Microcirc. 1972. 4: 1-77
25. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, editors. Chapter 7: Systematic reviews of etiology and risk. JBI manual for evidence synthesis, JBI. 2020. p.
26. Nania A, Granata F, Vinci S, Pitrone A, Barresi V, Morabito R. Necrosis score, surgical time, and transfused blood volume in patients treated with preoperative embolization of intracranial meningiomas. Analysis of a single-center experience and a review of literature. Clin Neuroradiol. 2014. 24: 29-36
27. Naqash IA, Draboo M, Lone AQ, Nengroo SH, Kirmani A, Bhat AR. Evaluation of acute normovolemic hemodilution and autotransfusion in neurosurgical patients undergoing excision of intracranial meningioma. J Anaesthesiol Clin Pharmacol. 2011. 27: 54-8
28. Neef V, König S, Monden D, Dubinski D, Benesch A, Raimann FJ. Clinical outcome and risk factors of red blood cell transfusion in patients undergoing elective primary meningioma resection. Cancers (Basel). 2021. 13: 3601
29. Nguyen HS, Janich K, Doan N, Patel M, Li L, Mueller W. Extent of T1+ C intensity is a predictor of blood loss in resection of meningioma. World Neurosurg. 2017. 101: 69-75
30. Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013. 15: ii1-56
31. O’Reilly RA, Hamilton RD. Acquired hemophilia, meningioma, and diphenylhydantoin therapy. J Neurosurg. 1980. 53: 600-5
32. Oka H, Kurata A, Kawano N, Saegusa H, Kobayashi I, Ohmomo T. Preoperative superselective embolization of skull-base meningiomas: Indications and limitations. J Neurooncol. 1998. 40: 67-71
33. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021. 88: 105906
34. Paleologos TS, Wadley JP, Kitchen ND, Thomas DG. Clinical utility and cost-effectiveness of interactive image-guided craniotomy: Clinical comparison between conventional and image-guided meningioma surgery. Neurosurgery. 2000. 47: 40-8
35. Perria C, Francaviglia N, Borzone M, Chinnici A, Piano E, Pacini P. The value and limitations of the CO2 laser in neurosurgery. Neurochirurgia (Stuttg). 1983. 26: 6-11
36. Rebai L, Mahfoudhi N, Fitouhi N, Daghmouri MA, Bahri K. Intraoperative tranexamic acid use in patients undergoing excision of intracranial meningioma: Randomized, placebo-controlled trial. Surg Neurol Int. 2021. 12: 289
37. Richter HP, Schachenmayr W. Preoperative embolization of intracranial meningiomas. Neurosurgery. 1983. 13: 261-8
38. Romani R, Silvasti-Lundell M, Laakso A, Tuominen H, Hernesniemi J, Niemi T. Slack brain in meningioma surgery through lateral supraorbital approach. Surg Neurol Int. 2011. 2: 167
39. Rutka J, Muller P, Chui M. Preoperative Gelfoam embolization of supratentorial meningiomas. Can J Surg J Can Chir. 1985. 28: 441-3
40. Saringcarinkul A, Chuasuwan S. Maximum surgical blood order schedule for elective neurosurgery in a University Teaching Hospital in Northern Thailand. Asian J Neurosurg. 2018. 13: 329
41. Sato H, Hyodo A, Matsumaru Y, Anno I, Kato T, Nose T. The evaluation of preoperative embolization of meningioma. Interv Neuroradiol. 1997. 3: 101-5
42. Sanai N, Sughrue ME, Shangari G, Chung K, Berger MS, McDermott MW. Risk profile associated with convexity meningioma resection in the modern neurosurgical era. J Neurosurg. 2010. 112: 913-9
43. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957. 20: 22-39
44. Song G, Li S, Wang X, Chang C, Chen J, Li H. Clinical analysis of preoperative embolization combined with Kawase approach in patients with petroclival meningioma. Biomed Res. 2018. 29: 1128-32
45. Sughrue ME, Rutkowski MJ, Shangari G, Chang HQ, Parsa AT, Berger MS. Risk factors for the development of serious medical complications after resection of meningiomas. J Neurosurg. 2011. 114: 697-704
46. Tarkkanen L, Lalla M, Laakso E, Troupp H. Changes in the requirements for blood transfusion in brain surgery. Acta Neurochir (Wien). 1981. 58: 213-9
47. Teitelbaum J, Fish D, Jaques L. Sudden onset of swelling and bleeding during meningioma surgery. Can J Neurol Sci. 2008. 35: 255-9
48. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D. PRISMA extension scoping reviews PRISMA-ScR: Checklist and Explanation. Ann Intern Med. 2018. 169: 467-73
49. Yadav YR, Parihar V, Agarwal M, Bhatele PR. Temporary clamping of external carotid artery in convexity, parasagittal and temporal base meningioma. Turk Neurosurg. 2012. 22: 44-9
50. Yang L, Wang HH, Wei FS, Ma LX. Evaluation of acute normovolemic hemodilution in patients undergoing intracranial meningioma resection: A quasi-experimental trial. Medicine (Baltimore). 2017. 96: e8093
51. Yew A, Trang A, Nagasawa DT, Spasic M, Choy W, Garcia HM. Chromosomal alterations, prognostic factors, and targeted molecular therapies for malignant meningiomas. J Clin Neurosci. 2013. 20: 17-22
52. Yin Y, Li Y, Jiang Z, Zhang C, Ge H, Chen Z. Clinical outcomes and complications of preoperative embolization for intracranial giant meningioma tumorectomy: A retrospective, observational, matched cohort study. Front Oncol. 2022. 12: 852327
53. Zuo MR, Liang RF, Li M, Xiang YF, Zhang SX, Yang Y. A comprehensive study of risk factors for post-operative pneumonia following resection of meningioma. BMC Cancer. 2019. 19: 100