- Department of Anesthesiology, Division of Neuroanaesthesia, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala, India.
- Department of Anesthesiology and Critical Care Medicine, Amrita Institute for Medical Sciences, Cochin, Kerala, India.
- Department of Anaesthesia and Intensive Care Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Hemant Bhagat
Department of Anaesthesia and Intensive Care Postgraduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_5_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ranganatha Praveen1, Aveek Jayant2, Shalvi Mahajan3, Kiran Jangra3, Nidhi Bidyut Panda3, Vinod K. Grover3, Manoj K. Tewari4, Hemant Bhagat3. Perioperative cardiovascular changes in patients with traumatic brain injury: A prospective observational study. 19-Apr-2021;12:174
How to cite this URL: Ranganatha Praveen1, Aveek Jayant2, Shalvi Mahajan3, Kiran Jangra3, Nidhi Bidyut Panda3, Vinod K. Grover3, Manoj K. Tewari4, Hemant Bhagat3. Perioperative cardiovascular changes in patients with traumatic brain injury: A prospective observational study. 19-Apr-2021;12:174. Available from: https://surgicalneurologyint.com/surgicalint-articles/10733/
Abstract
Background: Traumatic brain injury (TBI) is an acutely stressful condition. Stress and conglomeration of various factors predispose to the involvement of other organ systems. The stress response from TBI has been associated with cardiovascular complications reflecting as repolarization abnormalities on electrocardiogram (ECG) to systolic dysfunction on echocardiography. However, the perioperative cardiac functions in patients with TBI have not been evaluated.
Methods: We conducted a prospective observational study in 60 consecutive adult patients of either sex between the age of 10 and 70 years with an isolated head injury who were taken up for decompressive craniectomy as per institutional protocol. ECG and transthoracic echocardiography was carried out preoperatively and then postoperatively within 24–48 h.
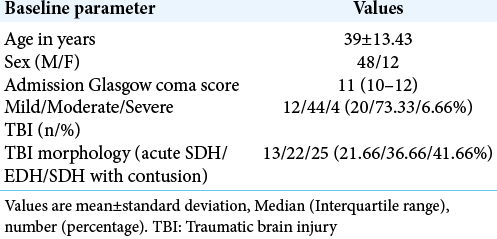
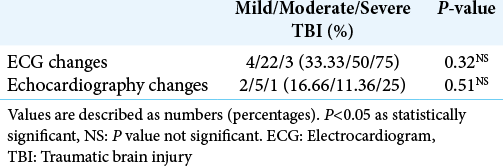
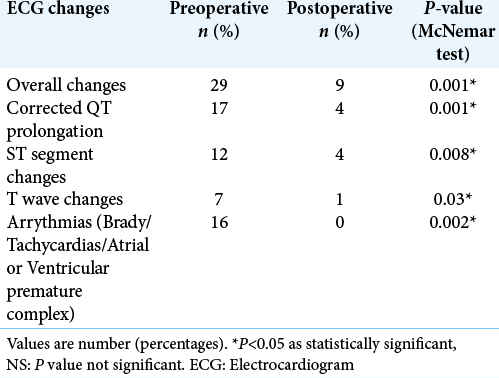
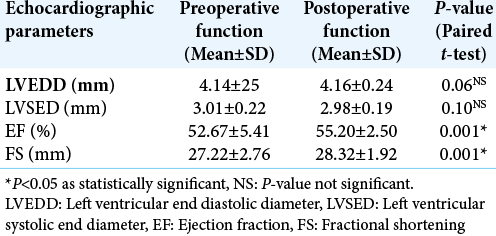
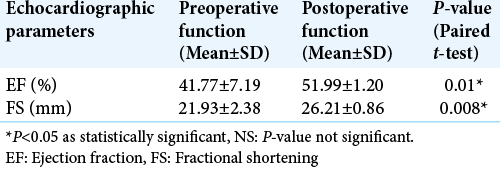
Results: The mean age of our study population was 39 + 13 years with a median Glasgow coma score of 11. A majority (73%) of our patients suffered moderate TBI. Preoperatively, ECG changes were seen in 48.33% of patients. Postoperatively, ECG changes declined and were seen only in 13.33% of the total patients. Similarly, echocardiography demonstrated preoperative systolic dysfunction in 13.33% of the total study population. Later, it was found that systolic function significantly improved in all the patients after surgery.
Conclusion: Cardiac dysfunction occurs frequently following TBI. Even patients with mild TBI had preoperative systolic dysfunction on echocardiography. Surgical intervention in the form of hematoma evacuation and decompression was associated with significant regression of both ECG and echocardiographic changes.
Keywords: Electrocardiography, Transthoracic echocardiography, Traumatic brain injury
INTRODUCTION
Acute insults to the brain during aneurysmal subarachnoid hemorrhage (aSAH), traumatic brain injury (TBI), and stroke have shown organ dysfunction extraneous to the central nervous system. The most common non-neurologic site involved was the respiratory system (23%) followed by the cardiovascular system (18%).[
The overwhelming focus has been on the clinical model of aSAH, electrocardiographic changes, and echocardiographic abnormalities in the literature.[
Head injury-related ECG abnormalities commonly include ST-segment changes, flat/inverted T waves, prominent U waves, and prolonged QTc interval.[
Few case reports have described the effect of surgical intervention on perioperative cardiovascular changes due to TBI.[
MATERIALS AND METHODS
This prospective, observational study was conducted on 60 consecutive isolated head injury patients of either sex between the age group of 10 and 70 years who underwent decompressive craniectomy over a period of 1½ year. All those patients who had valvular/coronary artery disease, or previous cardiac/lung surgery, and baseline electrolyte abnormality were excluded from the study. Before commencement of study, institute Ethics Committee approval (NK/1661/DM/10986) was sought and informed consent was obtained from either patients or next of the kin.
Cardiac evaluation
To carry out cardiac evaluation-ECG and transthoracic echocardiography were performed at two different points. Baseline ECG was obtained within the first 6 h after hospital admission, and then within 24–48 h after surgical intervention. Heart rate, rhythm, the QTc interval, and presence of repolarization abnormalities were computed and compared at two different time points.[
Transthoracic echocardiography was performed by cardiac anesthetist using P21x, 5-1MHz, 21 mm broadband, phased array probe of a Sonosite; Fujifilm Sonosite Inc. echocardiographic machine at baseline and then postoperatively within 24–48 h. Chamber quantification was performed as per the guidelines of the American Society of Echocardiography (ASE).[
Perioperative management
Patients underwent a brief detailed preanesthetic check in the preoperative holding area. All patients received general anesthesia as per the discretion of the attending neuroanesthesiologist. Standard American Society of Anesthesiologist intraoperative monitors were applied. Following surgical intervention, patients were shifted to the intensive care unit.
Statistical analysis
Normality of quantitative data such as age, admission Glasgow coma score (GCS), ECHO parameters (Left ventricular end diastolic diameter/Left ventricular systolic end diameter/EF/Fractional shortening [FS]) was checked by measures of Shapiro–Wilk test of normality. For normally distributed data, mean ± standard deviation (SD) whereas for skewed data, median, and interquartile range (IQR) has been reported. Paired t-test was applied to compare preoperative and postoperative normally distributed quantitative data and presented as mean ± SD. Categorical variables such as sex (M/F), GCS (Mild/moderate/severe TBI), TBI Morphology (acute SDH/EDH/SDH with contusion), and ECG/ECHO changes presented as number and percentages. Chi-square test (or Fisher Exact test, whichever applicable) was applied to find out significant association between preoperative ECG/Echocardiography changes and grades of TBI. McNemar test was applied to find out significant changes in categorical variables from preoperative to postoperative. All calculations were two sided and were performed using Statistical Package for the Social Sciences (SPSS Inc. 2013, version 22.0 for Windows, Armonk, NY, USA). P < 0.05 considered statistically significant.
RESULTS
Sixty consecutive subjects with isolated TBI requiring neurosurgical intervention, who satisfied the inclusion criteria were studied. Baseline demographic and clinical-radiological parameters are shown in [
[
[
A majority of patients with an ECG abnormality did not have a corresponding echocardiographic disturbance. Seven of the eight patients who had echocardiographic changes showed ECG changes. None of our patients received vasopressors/ inotropes postoperatively. Intraoperative hypotension was treated by phenylephrine boluses which occurred in few cases and were not statistically significant.
DISCUSSION
The brain-heart cross-talks have widely been studied in acute neurological insults such as aSAH and stroke.[
In this prospective study, it was found that 48.33% of patients with TBI have cardiovascular changes in a preoperative period as seen in ECG and 13% of patients have echocardiographic disturbances. The probable mechanism could be excessive circulating catecholamines to widespread inflammation, triggered by the neurological insult as suggested by the literature. Later, there was a gross decline in the cardiovascular changes in the postoperative period. Only 13.3% of patients showed ECG changes while none have echocardiography changes, respectively. This has been previously reported in various case-descriptions.[
In our study, repolarization abnormalities most commonly detected were QTc interval prolongation, T-wave inversion, and ST-segment changes. Krishnamoorthy et al. reported prolonged QTc, morphologic end repolarization abnormalities in 42.4% and 10.2% isolated TBI while ST changes, inverted T waves in 6.8% and 11.9% patients, respectively.[
In the reported literature, only a couple of studies have described the occurrence of echocardiographic changes in TBI patients. A study conducted by Krishnamoorthy et al. showed early systolic dysfunction in 22% moderate-severe TBI patients which improved over their 1st week while none in the mild TBI group demonstrated any changes.[
Prathep et al. in a retrospective analysis reported cardiac dysfunction in 22.3% of patients with TBI using echocardiography during the first 2 weeks. Reduced LVEF and RWMA were documented in 12% and 17.5% of patients, respectively.[
Venkata et al. found cardiac dysfunction in 13% patients (as mild-to-moderate reduction in LVEF) in moderate to severe TBI patients in the intensive care unit and patients who developed cardiac dysfunction had a higher proportion of ECG abnormalities.[
Hasanin et al. showed abnormal ECG changes in 62% and abnormal echocardiography findings in 28% patients in severe TBI.[
Limitations
Given the small number of patients in our study, a definitive conclusion will likely need replication in a larger subset of patients. Our study was not designed to build a regression model of the effect of surgery on non-neurological organ (systemic) effects of TBI; we can only suggest that this interesting phenomenon needs more detailed study. It further raises the interesting hypothesis that besides offering a therapeutic tool to address the primary insult, active surgical intervention may limit or treat secondary organ effects of neural injury. Nonmeasurement of cardiac enzymes was one of the few limitations of our study.
CONCLUSION
Cardiovascular abnormalities on ECG occurred in 48.33% and transthoracic echocardiographic changes in 13.33% of patients with TBI. Even patients with mild head injury develop echocardiographic changes indicating possible sudden ICP surge as the cause. Surgical decompression significantly improves cardiac functions. Considering that no in-hospital mortality occurred among any of our patient’s timed surgical intervention is a must. However, further study with a larger sample size is required to substantiate the same.
Ethics approval and consent to participate
Clearance to conduct the study was obtained from the Institute Ethics Committee in accordance with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from either patients or next of the kin all patients who participated in the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Ashok Kumar, Tutor, National Institute of Nursing Education (NINE), PGIMER, Chandigarh for his assistance with the statistical analysis.
References
1. Banki NM. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005. 112: 3314-9
2. Bhagat H, Chauhan H, Dash HH. ST elevation in a head-injured patient for emergency neurosurgery: Do we routinely need a cardiac evaluation?. Ann Card Anaesth. 2010. 13: 73-4
3. Bhagat H, Narang R, Sharma D, Dash HH, Chauhan H. ST elevation--an indication of reversible neurogenic myocardial dysfunction in patients with head injury. Ann Card Anaesth. 2009. 12: 149-51
4. Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008. 118: 397-409
5. Cheung RT, Hachinski V. Cardiac effects of stroke. Curr Treat Options Cardiovasc Med. 2004. 6: 199-207
6. Dash M, Bithal PK, Prabhakar H. ECG changes in pediatric patients with severe head injury. J Neurosurg Anesthesiol. 2003. 15: 270-3
7. Fan X, Du FH, Tian JP. The electrocardiographic changes in acute brain injury patients. Chin Med J (Engl). 2012. 125: 3430-3
8. Hasanin A, Kamal A, Amin S, Zakaria D, El Sayed R, Mahmoud K. Incidence and outcome of cardiac injury in patients with severe head trauma. Scand J Trauma Resusc Emerg Med. 2016. 24: 58
9. Jain A, Bhagat H, Kumar P. Electrocardiographic changes after severe head injury. Int J Cardiol. 2011. 151: 61-2
10. Jangra K, Grover VK, Bhagat H, Bhardwaj A, Tewari MK, Kumar B. Evaluation of the effect of aneurysmal clipping on electrocardiography and echocardiographic changes in patients with subarachnoid hemorrhage: A prospective observational study. J Neurosurg Anesthesiol. 2017. 29: 335-40
11. Kemp CD, Johnson JC, Riordan WP, Cotton BA. How we die: The impact of nonneurologic organ dysfunction after severe traumatic brain injury. Am Surg. 2008. 74: 866-72
12. Krishnamoorthy V, Rowhani-Rahbar A, Gibbons EF. Early systolic dysfunction following traumatic brain injury: A cohort study. Crit Care Med. 2017. 45: 1028-36
13. Krishnamoorthy V, Sharma D, Prathep S, Vavilala MS. Myocardial dysfunction in acute traumatic brain injury relieved by surgical decompression. Case Rep Anesthesiol. 2013. 2013: 482596
14. La Rocca R, Materia V, Pasquini A, La Rosa FC, Marte F, Patanè S. T-wave inversion after a severe head injury without ischemic heart disease. Int J Cardiol. 2011. 151: e43-4
15. Lanzino G, Kongable GL, Kassell NF. Electrocardiographic abnormalities after nontraumatic subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1994. 6: 156-62
16. Lim HB, Smith M. Systemic complications after head injury: A clinical review. Anaesthesia. 2007. 62: 474-82
17. Mittal M, Mahajan S. Post traumatic recurrent ventricular tachycardia in intensive care unit: It’s time not to give up. Indian J Anaesth. 2020. 64: 339-41
18. Nguyen H, Zaroff JG. Neurogenic stunned myocardium. Curr Neurol Neurosci Rep. 2009. 9: 486-91
19. Prathep S, Sharma D, Hallman M. Preliminary report on cardiac dysfunction after isolated traumatic brain injury. Crit Care Med. 2014. 42: 142-7
20. Singla SL, Jagdish , Garg P, Mehta RK. Electrocardiographic changes in craniocerebral trauma--could they serve as prognostic indicators?. J Indian Med Assoc. 2002. 100: 188-90
21. Venkata C, Kasal J. Cardiac dysfunction in adult patients with traumatic brain injury: A prospective cohort study. Clin Med Res. 2018. 16: 57-65
22. Wittebole X, Hantson P, Laterre PF. Electrocardiographic changes after head trauma. J Electrocardiol. 2005. 38: 77-81
23. Zaroff JG, Rordorf GA, Newell JB, Ogilvy CS, Levinson JR. Cardiac outcome in patients with subarachnoid hemorrhage and electrocardiographic abnormalities. Neurosurgery. 1999. 44: 34-40
24. Zygun D. Non-neurological organ dysfunction in neurocritical care: Impact on outcome and etiological considerations. Curr Opin Crit Care. 2005. 11: 139-43