- Department of Neurosurgery, Ehime University School of Medicine, Toon, Japan.
- Department of Rehabilitation, Ehime University Hospital, Toon, Japan.
- Division of Diagnostic Pathology, Ehime University Hospital, Toon, Japan.
Correspondence Address:
Akihiro Inoue, Department of Neurosurgery, Ehime University School of Medicine, Toon, Japan.
DOI:10.25259/SNI_495_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kosuke Kusakabe1, Akihiro Inoue1, Hideaki Watanabe1, Yawara Nakamura1, Masahiro Nishikawa1, Yoshihiro Ohtsuka1, Masahiro Ogura2, Seiji Shigekawa1, Mashio Taniwaki3, Riko Kitazawa3, Takeharu Kunieda1. Perioperative perampanel administration for early seizure prophylaxis in brain tumor patients. 11-Aug-2023;14:287
How to cite this URL: Kosuke Kusakabe1, Akihiro Inoue1, Hideaki Watanabe1, Yawara Nakamura1, Masahiro Nishikawa1, Yoshihiro Ohtsuka1, Masahiro Ogura2, Seiji Shigekawa1, Mashio Taniwaki3, Riko Kitazawa3, Takeharu Kunieda1. Perioperative perampanel administration for early seizure prophylaxis in brain tumor patients. 11-Aug-2023;14:287. Available from: https://surgicalneurologyint.com/surgicalint-articles/12493/
Abstract
Background: The efficacy of perioperative prophylactic antiepileptic drug therapy in “seizure-naïve” patients with brain tumor, including glioblastoma (GBM), remains controversial. This study investigated whether perampanel (PER) is effective and safe for preventing perioperative onset of epileptic seizures, so-called early seizure, in patients with brain tumors.
Methods: Forty-five patients underwent tumor resection through craniotomy for a primary supratentorial brain tumor at Ehime University Hospital between April 2021 and July 2022. PER was administered from the 1st to the 6th day after surgery for seizure prophylaxis. Occurrence of early seizure, hematological toxicities, and various side effects were recorded on postoperative days 7 and 14. In addition, the clinical course of these patients was compared with 42 brain tumor patients under the same treatment protocol who received levetiracetam (LEV) for seizure prophylaxis between April 2017 and October 2018.
Results: In 45 patients with brain tumor, including GBM, who received PER administration, no early seizures were identified within 7 days postoperatively. No adverse drug reactions such as hematological toxicity, liver or kidney dysfunction, or exanthematous drug eruption were observed in any cases. As side effects, somnolence was reported in 14 patients (31.1%), vertigo in 3 patients (6.7%), and headache in 3 patients (6.7%). Although somnolence and vertigo were difficult to assess in the case of intraparenchymal tumors, particularly GBM, these side effects were not identified in patients with extraparenchymal tumors such as meningiomas, epidermoid cysts, and pituitary adenomas. In addition, no significant differences were identified compared to patients who received LEV.
Conclusion: The efficacy and safety of PER in preventing early seizures among patients with brain tumors were retrospectively evaluated. Perioperative administration of PER to patients with brain tumors may reduce the risk of early seizures without incurring serious side effects, showing no significant differences compared to patients who received LEV.
Keywords: Brain tumor-related epilepsy, Early seizure, Glioblastoma, Perampanel, Side effect
INTRODUCTION
Perioperative prophylactic administration of antiepileptic drugs (AEDs) to patients without a history of seizures undergoing brain tumor resection is not recommended due to a lack of evidence but has been done routinely in many centers.[
Perampanel (PER) is a non-competitive α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate (AMPA) receptor antagonist that is clinically used for seizure control. In preclinical studies, PER has been found to be effective in preventing seizures, and this agent is expected to be particularly useful in controlling epileptic seizures associated with brain tumors, that is, brain tumor-related epilepsy (BTRE).[
MATERIALS AND METHODS
This study was approved by the Ethics Committee for Clinical Research at Ehime University Hospital (approval no. 2110012). All procedures were performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all individual participants included in the study.
Patients and study design
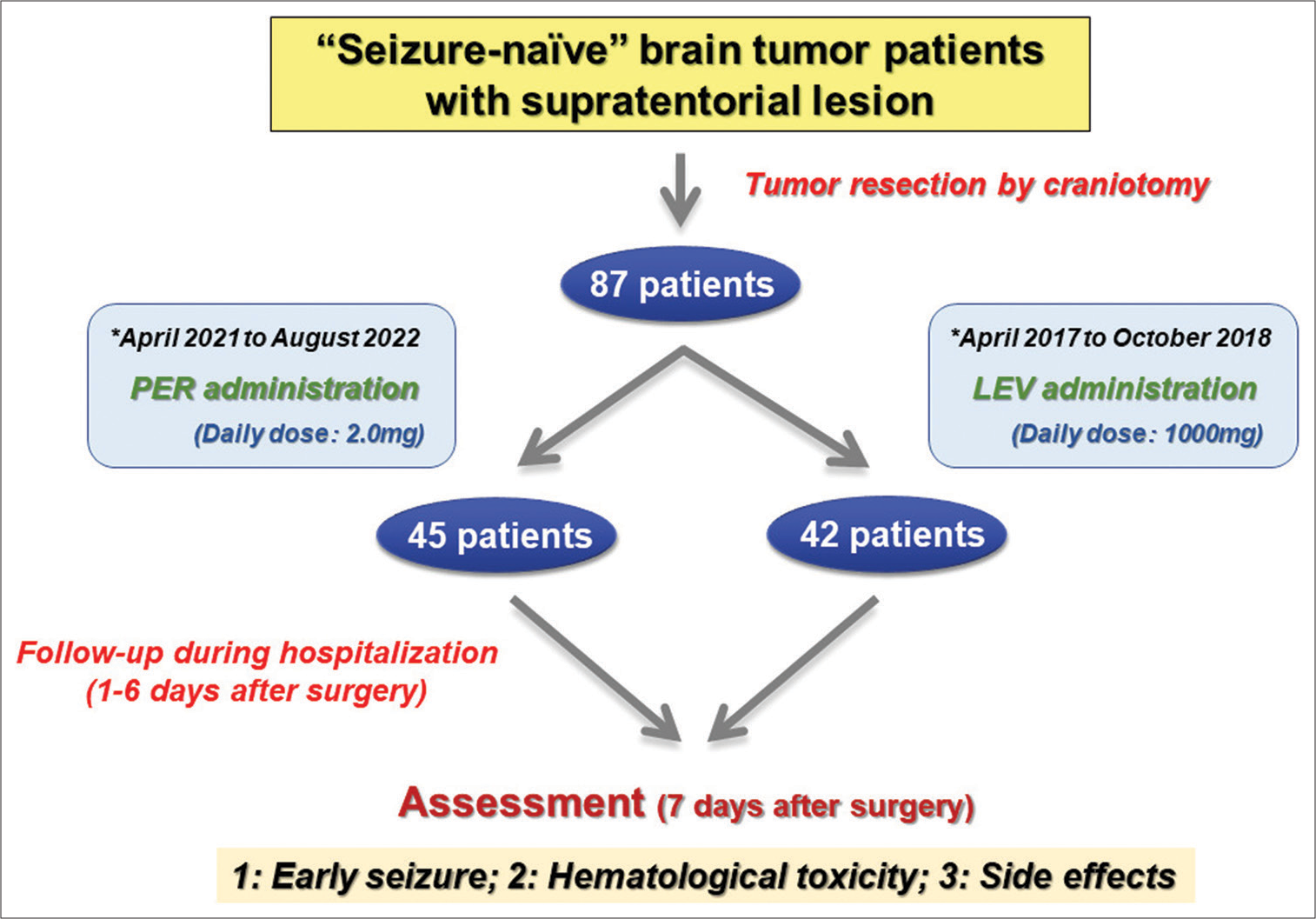
This study retrospectively enrolled 87 patients with brain tumor planned with inpatient treatment including surgical resection in the Department of Neurosurgery at Ehime University Hospital between April 2017 and August 2022. Among these patients, all 45 patients treated by Akihiro Inoue (Inoue. A) from April 2021 to August 2022 were administered PER perioperatively, while all 42 patients treated by Inoue A from April 2017 to October 2018 received LEV. Informed consent was obtained from all individual participants enrolled in the study. Specifically, participants were informed regarding the risk of the surgical procedure and the potential risks of microsurgery and chemoradiotherapy. This study included seizure-naïve adult (>18 years old) patients presenting with a radiologically suspected primary supratentorial brain tumor and all underwent craniotomy for tumor resection obtaining a histopathological diagnosis. Exclusion criteria comprised contraindications for PER or LEV (according to the relevant time period), and pre-existing administration of anticonvulsive medications. The total study duration for each patient was 15 days. The study design is shown in
Figure 1:
Study flow chart. During the study period, 87 “seizure-naïve” brain tumor patients were included in this study. Of these, 45 patients cured between April 2021 and August 2022 were administered perampanel during the perioperative period, ranging from 1 to 6 days after surgery, and 42 patients cured between April 2017 and October 2018 were administered levetiracetam during the same period for early seizure prophylaxis. At 7 days after surgery, we assessed the occurrence of early seizure and evaluated hematotoxicities and various side effects. PER: perampanel, LEV: levetiracetam.
PER and LEV administration, and assessment of early seizure
All enrolled patients with supratentorial brain tumor were administered oral PER or LEV during the perioperative period for a total of 6 days after surgery. In addition, all patients did not receive any preoperative AEDs including PER and LEV. The dose was 2.0 mg/day for PER and 1000 mg/day for LEV. All patients were hospitalized at Ehime University Hospital for ≥14 days after surgery and were evaluated for the occurrence of early seizures during the hospitalization period. In this study, early seizure was defined as epilepsy occurring within 1 week after surgery. If early seizures were suspected clinically, electroencephalography was performed. In addition, in cases with intraparenchymal tumors, cognitive function was assessed by mini-mental state examination (MMSE) preoperatively and postoperative day 14.
Evaluation of hematological toxicity and various side effects
Hematological laboratory markers including neutrophils, platelets, hemoglobin, lymphocytes (total), and presence of febrile neutropenia were evaluated on postoperative days 7 and 14 for all enrolled patients. Self-reported side effects were also elicited during hospitalization. All patients underwent routine magnetic resonance imaging (MRI) at least once within 5 days postoperatively to rule out postoperative complications such as bleeding or ischemia and to depict the amount of resection.
Statistical analysis
Values are expressed as the mean ± standard deviation, and data were compared using the two-tailed Student’s t-test (unpaired) and the chi-square test. Significance was set for values of P < 0.05. All analyses were performed using Office Excel 2016 software (Microsoft, Redmond, WA, USA).
RESULTS
Patient characteristics
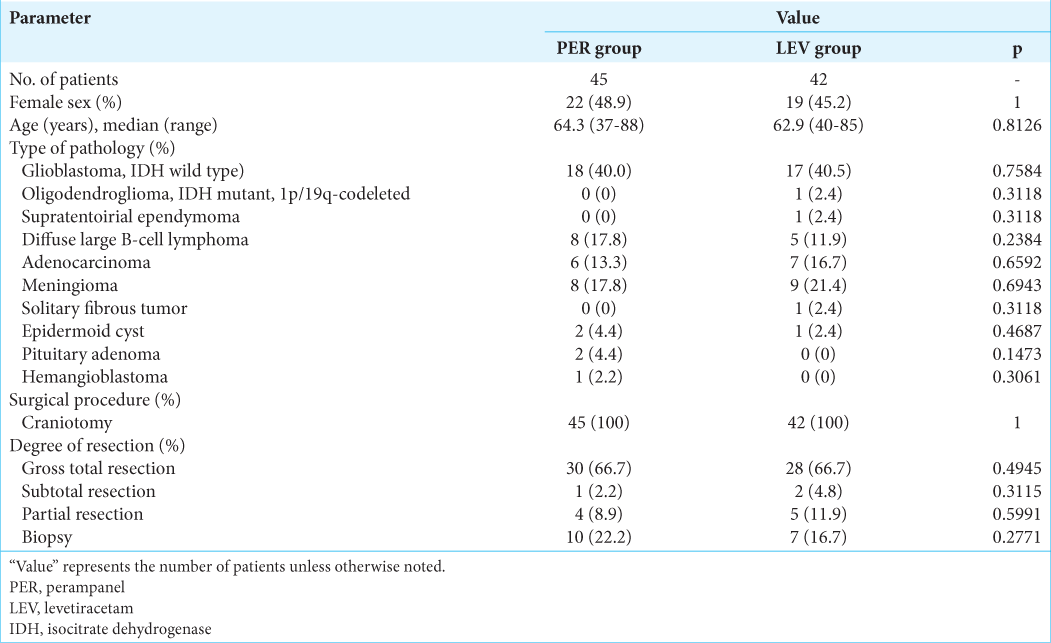
Among the 87 patients with intracranial brain tumor enrolled at our institution during the study period, 45 subjects prophylactically received PER, and 42 received LEV during the perioperative period. All seizure-naïve patients with supratentorial brain tumor for whom MRI could be performed were enrolled in this study. All patients underwent the same protocol of microsurgical resection by echo-linked navigation-guided microsurgery using MRI and methionine-positron emission tomography fusion images and fence-post catheter technique. [
Occurrence of early seizure and adverse events (AEs)
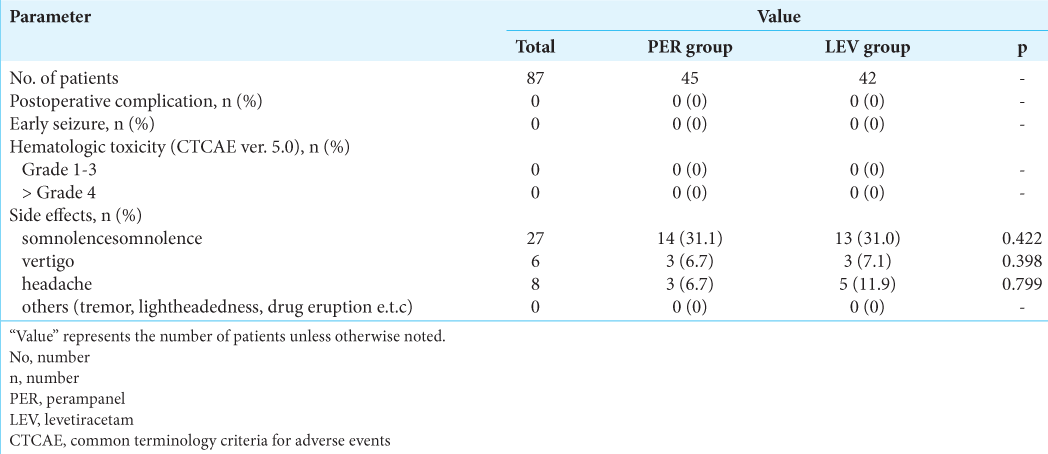
In this study, all 87 patients underwent craniotomy performed by the same surgeon (Inoue. A) under the same protocol using image-guided navigation. Computed tomography was performed immediately after surgery and the day after surgery, and MRI was performed within 10 days of craniotomy, with no obvious postoperative hemorrhage or extensive ischemic infarction observed. In addition, no severe infections, unexpected postoperative complications, or postoperative psychotic symptoms that would have caused a decrease in KPS were identified. In terms of epileptic events, early seizures were not recognized in any of the 87 brain tumor patients during the 14-day observation period [
Analysis of hematological toxicity
To determine hematotoxicities associated with PER or LEV administration, we used the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE ver. 5.0) classification of hematotoxic AEs. We assessed laboratory examinations at two points on postoperative days 7 and 14 in line with CTCAE ver. 5.0 criteria. According to this classification, no hematological AEs were categorized as Grade 4 or reported as related to PER or LEV administration in any of the patients in this study. In addition, no hematotoxicities classified as Grade 1–3 were observed in patients during this follow-up period. No hepatic or renal dysfunction was recognized in any cases either [
Assessment of various side effects
A precise frequencies of each side effects and their severities over the entire study period can be found below. In the PER group, somnolence was the most frequent AE, as reported in 14 patients (31.1%). Vertigo was reported in 3 patients (6.7%) and headache in 3 patients (6.7%). In the LEV group, somnolence was seen in 13 patients (31.0%), vertigo in 3 patients (7.1%), and headache in 5 patients (11.9%). However, across the two groups, no patients reported other side effects, such as tremor, lightheadedness, and exanthematous drug eruption. While somnolence and lightheadedness were difficult to assess in cases of intraparenchymal tumors such as malignant glioma, these side effects were not identified in extraparenchymal tumor patients such as those with meningioma, pituitary adenoma, epidermoid, or hemangioblastoma.
DISCUSSION
Epileptic seizures associated with brain tumors, so-called BTRE, can cause motor and cognitive impairments such as Todd’s palsy that can significantly impair patient QOL. The incidence of BTRE is as high as 40–60%, often representing the first clinical manifestation of tumor, and providing a sign of progression or recurrence.[
Although the efficacy of AEDs in preventing early seizure remains controversial, we have administered AEDs postoperatively in all patients unless a history of adverse effects was present. These seizures are classified as early posttraumatic seizure (PTS) caused by the surgical procedure. We, therefore, speculated that the choice of AEDs for prophylaxis of postoperative early seizure may be appropriate for treatment similar to the prophylaxis of early PTS. The guidelines for severe traumatic brain injury based on the Brain Trauma Foundation recommend prophylactic administration of phenytoin,[
Recently, glutamate has been featured as a key excitatory neurotransmitter in the brain, and excessive glutamate release and receptor overactivation are thought to be involved in the neurological damage caused by traumatic brain injury.[
We only have small preliminary data of our own on blood concentrations; however, they reach the optimal range (50–400 ng/mL) within 3 days after administration of PER (2.0 mg/day) (unpublished data). In the present study, no cases of early seizure were identified in the PER group up to postoperative day 7, even in patients with intra-axial tumors such as malignant glioma and malignant lymphoma. In addition, no serious hematotoxicities more than Grade 2 of CTCAE ver. 5.0 were seen, and also, no cognitive decline has been observed. However, a small number of side effects such as somnolence, headache, and dizziness appeared. As in the PER group, the LEV group showed no occurrence of early seizure and no significant AEs in the form of hematotoxicities. However, as in the PER group, side effects such as somnolence, headache, and dizziness were identified. As a result, no significant differences were apparent between PER and LEV groups in terms of side effects (P > 0.05). The present cohort showed no significant differences in age, sex, KPS score, pathological findings, or IDH-1 mutational status (as assessed by Sanger sequencing) between the groups. We likewise detected no significant differences in the extent of resection between GTR and non-GTR groups (i.e., STR, PR, or biopsy). Taken together, perioperative administration of PER to “seizure-naïve” brain tumor patients may reduce the risk of early seizures without serious side effects, showing no significant differences compared to patients who received LEV. This, in turn, may lead to favorable QOL and smooth introduction of chemotherapy and other treatments.
Several limitations to the present study must be kept in mind. This study was conducted by analyzing data from a relatively small number of patients, which may reflect the difficulty of enrolling sufficient numbers of patients with brain tumor-administered PER, a novel AED, perioperatively from only a single center. A control group (no administration of AED) was unavailable, as our institution routinely applied perioperative AEDs for tumor resection. A more extensive analysis with a larger number of patients is needed to obtain definitive conclusions before the proposed modality can be considered truly useful in clinical practice.
CONCLUSION
Perioperative administration of PER to “seizure-naïve” patients with brain tumor, particularly malignant glioma, may reduce the risk of perioperative early seizures without serious hematotoxicities or side effects, showing no significant differences in terms of the effectiveness of seizure control and safety compared to LEV. Also considering its good BBB permeability of PER, these findings may represent a new therapeutic strategy not only for brain tumor treatment but also for every other craniotomy in the field of neurosurgery. This may also lead to better therapeutic planning and an improved clinical course for patients with brain tumor, including malignant glioma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We are grateful to Taichi Furumochi and Yasuhiro Shiraishi from the Department of Neurology and to Eiji Katayama, Takuya Kondo and Satsuki Myoga from the Department of Pathology at Ehime University Hospital, Japan, for their help in obtaining radiological imaging and pathological findings.
References
1. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, editors. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017. 80: 6-15
2. Chen T, Liu WB, Qian X, Xie KL, Wang YH. The AMPAR antagonist perampanel protects the neurovascular unit against traumatic injury via regulating Sirt3. CNS Neurosci Ther. 2021. 27: 134-44
3. Cucchiara F, Pasqualetti F, Giorgi FS, Danesi R, Bocci G. Epileptogenesis and oncogenesis: An antineoplastic role for antiepileptic drugs in brain tumours?. Pharmacol Res. 2020. 156: 104786
4. Dewan MC, Thompson RC, Kalkanis SN, Barker FG, Hadjipanayis CG. Prophylactic antiepileptic drug administration following brain tumor resection: Results of a recent AANS/CNS Section on Tumors survey. J Neurosurg. 2017. 126: 1772-8
5. Dewan MC, White-Dzuro GA, Brinson PR, Zuckerman SL, Morone PJ, Thompson RC. The influence of perioperative seizure prophylaxis on seizure rate and hospital quality metrics following glioma resection. Neurosurgery. 2017. 80: 563-70
6. Ersoy TF, Ridwan S, Grote A, Coras R, Simon M. Early postoperative seizures (EPS) in patients undergoing brain tumour surgery. Sci Rep. 2020. 10: 13674
7. Glantz MJ, Cole BF, Forsyth PA, Recht LD, Wen PY, Chamberlain MC. Practice parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000. 54: 1886-93
8. Hibi S, Ueno K, Nagato S, Kawano K, Ito K, Norimine Y. Discovery of 2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile (perampanel): A novel, noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) receptor antagonist. J Med Chem. 2012. 55: 10584-600
9. Inaba K, Menaker J, Branco BC, Gooch J, Okoye OT, Herrold J. A prospective multicenter comparison of levetiracetam versus phenytoin for early posttraumatic seizure prophylaxis. J Trauma Acute Care Surg. 2013. 74: 766-71
10. Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002. 8: 971-8
11. Izumoto S, Miyauchi M, Tasaki T, Okuda T, Nakagawa N, Nakano N. Seizures and tumor progression in glioma patients with uncontrollable epilepsy treated with perampanel. Anticancer Res. 2018. 38: 4361-6
12. Konrath E, Marhold F, Kindler W, Scheichel F, Popadic B, Blauensteiner K. Perioperative levetiracetam for seizure prophylaxis in seizure-naive brain tumor patients with focus on neurocognitive functioning. BMC Neurol. 2022. 22: 250
13. Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J. Randomized phase III study 306: Adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012. 78: 1408-15
14. Lee CH, Koo HW, Han SR, Choi CY, Sohn MJ, Lee CH. Phenytoin versus levetiracetam as prophylaxis for postcraniotomy seizure in patients with no history of seizures: Systematic review and meta-analysis. J Neurosurg. 2019. 130: 1-8
15. Lockney DT, Vaziri S, Walch F, Kubilis P, Neal D, Murad GJ. Prophylactic antiepileptic drug use in patients with brain tumors undergoing craniotomy. World Neurosurg. 2017. 98: 28-33
16. Luo P, Fei F, Zhang L, Qu Y, Fei Z. The role of glutamate receptors in traumatic brain injury: Implications for postsynaptic density in pathophysiology. Brain Res Bull. 2011. 85: 313-20
17. Nakamura Y, Inoue A, Nishikawa M, Ohnishi T, Yano H, Kanemura Y. Quantitative measurement of peritumoral concentrations of glutamate, N-acetyl aspartate, and lactate on magnetic resonance spectroscopy predicts glioblastoma-related refractory epilepsy. Acta Neurochir (Wien). 2022. 164: 3253-66
18. Nicolas JM, Hannestad J, Holden D, Kervyn S, Nabulsi N, Tytgat D. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia. 2016. 57: 201-9
19. Ohue S, Kohno S, Inoue A, Yamashita D, Harada H, Kumon Y. Accuracy of diffusion tensor magnetic resonance imaging-based tractography for surgery of gliomas near the pyramidal tract: A significant correlation between subcortical electrical stimulation and postoperative tractography. Neurosurgery. 2012. 70: 283-93
20. Ohue S, Kohno S, Inoue A, Yamashita D, Matsumoto S, Suehiro S. Surgical results of tumor resection using tractography-integrated navigation-guided fence-post catheter techniques and motor-evoked potentials for preservation of motor function in patients with glioblastomas near the pyramidal tracts. Neurosurg Rev. 2015. 38: 293-306
21. Okita Y, Nonaka M, Shofuda T, Kanematsu D, Yoshioka E, Kodama Y. (11)C-methinine uptake correlates with MGMT promoter methylation in nonenhancing gliomas. Clin Neurol Neurosurg. 2014. 125: 212-6
22. Oushy S, Sillau SH, Ney DE, Damek DM, Youssef AS, Lillehei KO. New-onset seizure during and after brain tumor excision: A risk assessment analysis. J Neurosurg. 2018. 128: 1713-8
23. Pourzitaki C, Tsaousi G, Apostolidou E, Karakoulas K, Kouvelas D, Amaniti E. Efficacy and safety of prophylactic levetiracetam in supratentorial brain tumour surgery: A systematic review and meta-analysis. Br J Clin Pharmacol. 2016. 82: 315-25
24. Rösche J, Piek J, Hildebrandt G, Grossmann A, Kirschstein T, Benecke R. Perampanel in the treatment of a patient with glioblastoma multiforme without IDH1 mutation and without MGMT promotor methylation. Fortschr Neurol Psychiatr. 2015. 83: 286-9
25. Shin HJ, Lee KY, Kang JW, Choi SG, Kim DW, Yi YY. Perampanel reduces brain damage via induction of M2 microglia in a neonatal rat stroke model. Int J Nanomedicine. 2022. 17: 2791-804
26. Tong X, Patsalos PN. A microdialysis study of the novel antiepileptic drug levetiracetam: Extracellular pharmacokinetics and effect on taurine in rat brain. Br J Pharmacol. 2001. 133: 867-74
27. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: New insights and evidence-based management. Oncologist. 2014. 19: 751-9
28. Walbert T, Harrison RA, Schiff D, Avila EK, Chen M, Kandula P. SNO and EANO practice guideline update: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro Oncol. 2021. 23: 1835-44
29. Yu T, Teng ZT, Liu XY, Wang H. Effectiveness of perampanel in the treatment of pediatric patients with focal epilepsy and ESES: A single-center retrospective study. Front Pharmacol. 2022. 13: 1026836