- Department of Neurosurgery, University of Colorado School of Medicine, Aurora, Colorado, United States.
Correspondence Address:
Jessa E. Hoffman, Department of Neurosurgery, University of Colorado School of Medicine, Aurora, Colorado, United States.
DOI:10.25259/SNI_482_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jessa E. Hoffman, Brent Morel, Blake Wittenberg, David Kumpe, Joshua Seinfeld, Zach Folzenlogen, David Case, Robert Neumann, Luis Cava, Robert Breeze, Laura Wiley, Christopher Roark. Periprocedural management of ruptured blister aneurysms treated with pipeline flow diversion. 08-Mar-2024;15:73
How to cite this URL: Jessa E. Hoffman, Brent Morel, Blake Wittenberg, David Kumpe, Joshua Seinfeld, Zach Folzenlogen, David Case, Robert Neumann, Luis Cava, Robert Breeze, Laura Wiley, Christopher Roark. Periprocedural management of ruptured blister aneurysms treated with pipeline flow diversion. 08-Mar-2024;15:73. Available from: https://surgicalneurologyint.com/surgicalint-articles/12793/
Abstract
Background: Blister aneurysms are high-risk intracranial vascular lesions. Definitive treatment of these lesions has been challenging. Severe disability or mortality rates are as high as 55% when these lesions are treated with open surgery. Recent data show that flow diversion is a safe and effective alternative treatment for blister aneurysms. Rerupture of the functionally unsecured lesion remains a concern as flow diversion does not immediately exclude the aneurysm from the circulation.
Methods: A retrospective review was performed of any patients with ruptured blister aneurysms treated with a pipeline embolization device between 2010 and 2020 at the University of Colorado.
Results: In this paper, we present the results of the intensive care management of ruptured intracranial blister aneurysms after flow-diverting stent placement.
Conclusion: Despite the need for dual antiplatelet therapy and the delayed occlusion of blister aneurysms treated with flow diversion, we did not find an increase in periprocedural complications.
Keywords: Blister aneurysm, Flow diversion, Pipeline
INTRODUCTION
Blister aneurysms are classically defined as half-dome-shaped lesions of the supraclinoid internal carotid artery (ICA).[
Recent studies provide evidence for flow-diverting stent placement as a promising option for blister aneurysms that are not safe for immediate obliteration through microsurgery or coil embolization.[
The hospital management of patients with aneurysmal subarachnoid hemorrhage (aSAH) is complicated by placing a flow-diverting stent. Patients often require external ventricular drain (EVD) placement and other invasive procedures after aSAH. Patients on dual antiplatelet therapy (DAPT) are at higher risk of hemorrhagic complications during these procedures.[
MATERIALS AND METHODS
Patient data
We conducted an IRB-approved retrospective review of patients who presented to the University of Colorado Hospital (UCH) between 2010 and 2020. This review included all patients with SAH from a ruptured blister aneurysm treated with a pipeline embolization device (PED; Medtronic, Minneapolis, Minnesota). All PEDs used were the flex model except one case of retreatment with a Pipeline Classic Embolization Device (Medtronic, Minneapolis, Minnesota). We defined ruptured blister aneurysms as sessile aneurysms arising from non-branching points of the supraclinoid ICA with SAH. Aneurysm data collected through chart review included initial patient demographics, Hunt and Hess (HH) grade, aneurysm characteristics, and aneurysm treatment. Chart review also included intensive care unit (ICU) course and medications, subsequent procedures, complications, mRS at discharge, and follow-up imaging.
RESULTS
Demographics and aneurysm characteristics
Forty-three patients presented to UCH with possible blister aneurysms based on any imaging modality. We excluded patients if there was no treatment at UCH if digital subtraction angiography (DSA) failed to identify a blister aneurysm, or if there was no rupture event. Seventeen patients had a confirmed ruptured supra clinoid ICA blister aneurysm treated at our hospital. Of these, microsurgery was performed in two: one patient had balloon-assisted coiling, one patient had multiple stents placed, and stent-assisted coiling was performed in one patient. Twelve patients (eight females and four males) were treated with a PED and included in this series.
The average age at presentation for patients treated with PED was 49.8 years old (range 28–74), with an average HH score of 2.9 [
Aneurysm treatment
Microsurgery
Two patients had blister aneurysms treated with open surgery. One underwent a clip wrap of a right ICA blister aneurysm. The second patient underwent clip-trapping of a left ICA blister aneurysm. Both patients suffered large territory strokes and developed malignant intracranial HTN requiring decompressive craniectomy. Discharge modified Rankin score (mRS) was 5 in both patients.
Traditional endovascular therapy
Three patients had ruptured blister aneurysms treated with stents and/or coils. One patient had balloon-assisted coiling with intraprocedural left M3 thrombus, leading to stroke and edema requiring decompressive hemicraniectomy. This patient also had an aneurysm recurrence, which was retreated with pipeline coiling. Per the patient’s goal of care to be independent, the patient’s family decided to withdraw care after a lack of improvement in their exam. One patient had two Enterprise stents (Codman, Raynham, Massachusetts) placed initially. On follow-up, DSA growth of the blister aneurysm was noted. Retreatment with coiling led to intraprocedural aneurysmal rupture followed by successful occlusion of the aneurysm. This patient was discharged with mRS of 3. Stent-assisted coiling was performed in one patient who had growth of the aneurysm requiring further treatment with coils. This patient was discharged with mRS of 4. The two surviving patients did not have any residual aneurysmal filling.
PED therapy
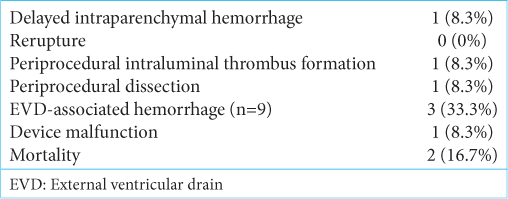
Twelve patients had blister aneurysms treated with PED placement. All 12 patients had successful initial PED placement. Two of these patients had two PEDs placed during the initial treatment. The remaining ten patients had a single PED placed at initial treatment. One patient required retreatment after the initial pipeline placement. There were no intraprocedural deaths or intraprocedural hemorrhagic events.
There were two deaths following treatment with PED (mortality rate: 16.7%) [
One patient was re-treated after the initial PED Flex placement. The operator attempted to use Pipeline Classic for the second treatment. This treatment followed DSA on postprocedure day 4, which documented continued growth of the aneurysm. At the time of the second treatment, there was limited availability of a compatible microcatheter system for the Pipeline Flex. During this procedure, the Pipeline Classic stent failed to open. This event led to the fracture of the stent support wire and retention of the unexpanded embolization device in the MCA and subsequent right MCA infarction. Follow-up DSA showed no residual aneurysm filling. The patient experienced left-sided neglect but no other clinical symptoms.
One patient had intraluminal non-occlusive stent thrombosis on follow-up angiography. This thrombosis was treated with heparin therapy, which led to the resolution of the thrombus. There was no clinical or imaging evidence of infarct throughout this patient’s hospital course.
One patient had increased technical complexity of PED placement as the PED did not fully deploy and required contralateral femoral puncture and retrograde access from the contralateral ICA to achieve patency. No thrombosis or infarcts occurred from this procedure. This patient had a pre-existing dissection of the bilateral cervical ICA, intraprocedural dissection of the ipsilateral cervical ICA, and pseudoaneurysm of the contralateral cervical ICA, neither of which required further procedural intervention.
No patients treated with flow diversion in this series suffered a re-rupture of the aneurysm.
Radiographic and clinical outcomes
After the initial treatment, all 12 aneurysms were rated a three on the Raymond–Roy Occlusion scale (Class 1: Complete obliteration, Class 2: Residual neck filling, Class 3: Residual aneurysm filling)[
Follow-up imaging consists of a DSA except in one patient, followed by magnetic resonance angiography (MRA) due to difficult access and a history of cervical carotid pseudoaneurysm. Raymond–Roy Occlusion rates of surviving patients with at least three months of follow-up imaging are as follows: eight patients were rated grade 1, and one patient was rated grade 2. No patients remained in grade 3. The occlusion rate was 88.9%. The follow-up period averaged 11.6 months (range: 3–20). One patient required retreatment with another PED for a retreatment rate of 8.3%.
We collected mRS outcomes at the time of discharge from the hospital. Five patients had an mRS of one. Three patients had an mRS of three. One patient had an mRS of four. One patient had an mRS of five. Two patients died. The five patients who presented with a low HH score had an average mRS of 1.4. Patients who presented with a high HH score had an average mRS of 4.
Critical care results
All patients were monitored in the ICU for at least 21 days following hemorrhage. All 12 patients treated with PED had a goal systolic blood pressure (SBP) of 140 mmHg or less until PED placement. One patient transitioned to comfort care due to a concurrent left MCA stroke. Ten of the 11 surviving patients had radiographic evidence of vasospasm. Computed tomography angiogram diagnosed vasospasm in all ten of these patients. DSA confirmed this diagnosis. We treated vasospasm with the administration of intra-arterial verapamil. Six of these patients required repeat treatment over several days. Three patients had symptomatic vasospasm measured by examination changes of new-onset weakness, numbness, or mental status deterioration.
During the vasospasm window, the average maximum SBP goal was 162 mmHg (range 140–220 mmHg). We kept the SBP goal at 140 mmHg in four patients due to a presumed higher risk of rupture based on angiography. The remaining eight patients had a maximum blood pressure (BP) goal of at least 150 mmHg. The average maximum SBP reached was 189 mmHg (Range 157–241 mmHg). The average amount of days spent in permissive HTN was 11.75. Four patients were treated with vasopressors to augment their BP during the vasospasm window. There were no re-rupture events.
We loaded all 12 patients with DAPT before PED placement. All 12 patients received aspirin after PED placement. The second antiplatelet agent used in all cases was clopidogrel, except for one patient maintained on ticagrelor. Nine patients had EVDs present at some point in their ICU stay.
Three patients had evidence of EVD-associated hemorrhage while on DAPT. One patient had an EVD tract bleed before any dual antiplatelet administration, which enlarged after PED placement and initiation of DAPT. One patient had an EVD tract hemorrhage develop at the time of PED placement. One patient had a tract hemorrhage after the removal of the EVD. There was an overall rate of 33% for any EVD tract hemorrhage or increase in existing tract hemorrhage. None of the EVD tract hemorrhages were clinically relevant or required surgical intervention. A single patient had a lumbar drain placed for CSF diversion. There was no evidence of complication or bleeding in this patient.
DISCUSSION
In this study, we describe our 10-year experience with PED placement to treat ruptured blister aneurysms. Our overall mortality rate of 16% is a marked improvement over the historical mortality rate for the microsurgical treatment of these lesions. The poor outcomes in open surgical intervention for blister lesions led to a search for effective endovascular intervention. Definitive treatment with stents, coils, or both have a high risk of morbidity and intraprocedural rupture.[
One complicating factor for ICU patients with PED placement is the increased risk of bleeding secondary to necessary DAPT. The 33% rate of EVD tract hemorrhage in this study is consistent with rates found in other critical care studies showing EVD hemorrhage in the setting of DAPT.[
The second issue arising from PED placement is the lack of immediate occlusion of the ruptured aneurysm.[
CONCLUSION
To the best of our knowledge, this is the first paper to discuss the implications of pipeline placement in the periprocedural ICU setting. Despite the need for DAPT and the delayed occlusion of blister aneurysms treated with flow diversion, we did not find an increase in ICU complications.
Ethical approval
The research/study approved by the Institutional Review Board at the University of Colorado, number 18-1770, dated 3/25/2022.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abe M, Tabuchi K, Yokoyama H, Uchino A. Blood blisterlike aneurysms of the internal carotid artery. J Neurosurg. 1998. 89: 419-24
2. Awad IA, Carter LP, Spetzler RF, Medina M, Williams FC. Clinical vasospasm after subarachnoid hemorrhage: Response to hypervolemic hemodilution and arterial hypertension. Stroke. 1987. 18: 365-72
3. Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: Five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. 2017. 80: 40-8
4. Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009. 40: 994-1025
5. Bulsara KR, Kuzmik GA, Hebert R, Cheung V, Matouk CC, Jabbour P. Stenting as monotherapy for uncoilable intracranial aneurysms. Neurosurgery. 2013. 73: S80-5
6. Chalouhi N, Zanaty M, Tjoumakaris S, Gonzalez LF, Hasan D, Kung D. Treatment of blister-like aneurysms with the pipeline embolization device. Neurosurgery. 2014. 74: 527-32
7. Ecker A, Riemenschneider PA. Arteriographic demonstration of spasm of the intracranial arteries, with special reference to saccular arterial aneurysms. J Neurosurg. 1951. 8: 660-7
8. Gardner PA, Engh J, Atteberry D, Moossy JJ. Hemorrhage rates after external ventricular drain placement. J Neurosurg. 2009. 110: 1021-5
9. Hudson JS, Prout BS, Nagahama Y, Nakagawa D, Guerrero WR, Zanaty M. External ventricular drain and hemorrhage in aneurysmal subarachnoid hemorrhage patients on dual antiplatelet therapy: A retrospective cohort study. Neurosurgery. 2019. 84: 479-84
10. Kan P, Sweid A, Srivatsan A, Jabbour P. Expanding indications for flow diverters: Ruptured aneurysms, blister aneurysms, and dissecting aneurysms. Neurosurgery. 2020. 86: S96-103
11. Kassell NF, Peerless SJ, Durward QJ, Beck DW, Drake CG, Adams HP. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery. 1982. 11: 337-43
12. Kosnik EJ, Hunt WE. Postoperative hypertension in the management of patients with intracranial arterial aneurysms. J Neurosurg. 1976. 45: 148-54
13. Linfante I, Mayich M, Sonig A, Fujimoto J, Siddiqui A, Dabus G. Flow diversion with Pipeline Embolic Device as treatment of subarachnoid hemorrhage secondary to blister aneurysms: Dual-center experience and review of the literature. J Neurointerv Surg. 2017. 9: 29-33
14. Mazur MD, Taussky P, MacDonald JD, Park MS. Rerupture of a blister aneurysm after treatment with a single flow-diverting stent. Neurosurgery. 2016. 79: E634-8
15. Meling TR, Sorteberg A, Bakke SJ, Slettebø H, Hernesniemi J, Sorteberg W. Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: Treatment and outcome. J Neurosurg. 2008. 108: 662-71
16. Miller C, Tummala RP. Risk factors for hemorrhage associated with external ventricular drain placement and removal. J Neurosurg. 2017. 126: 289-97
17. Nasra M, Mitreski G, Kok HK, Maingard J, Slater LA, Russell JH. Contemporary treatment of intracranial blood blister aneurysms-a systematic review. J Stroke Cerebrovasc Dis. 2021. 30: 105968
18. Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the surpaclinoid portion of the internal carotid artery: Internal carotid artery trunk aneurysms. Neurosurgery. 2000. 47: 578-83
19. Ohara H, Sakamoto T, Suzuki J, editors. Sclerotic cerebral aneurysms. Cerebral aneurysms. Tokyo: Neuron; 1979. p. 673-82
20. Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001. 32: 1176-80
21. Otsubo H, Takemae T, Inoue T, Kobayashi S, Sugita K. Normovolaemic induced hypertension therapy for cerebral vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien). 1990. 103: 18-26
22. Peitz GW, Sy CA, Grandhi R. Endovascular treatment of blister aneurysms. Neurosurg Focus. 2017. 42: E12
23. Peschillo S, Cannizzaro D, Caporlingua A, Missori P. A systematic review and meta-analysis of treatment and outcome of blister-like aneurysms. AJNR Am J Neuroradiol. 2016. 37: 856-61
24. Rouchaud A, Brinjikji W, Cloft HJ, Kallmes DF. Endovascular treatment of ruptured blister-like aneurysms: A systematic review and meta-analysis with focus on deconstructive versus reconstructive and flow-diverter treatments. AJNR Am J Neuroradiol. 2015. 36: 2331-9
25. Rowe AS, Rinehart DR, Lezatte S, Langdon JR. Intracerebral hemorrhage after external ventricular drain placement: An evaluation of risk factors for post-procedural hemorrhagic complications. BMC Neurol. 2018. 18: 22
26. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001. 32: 1998-2004
27. Russin JJ, Kramer DR, Thomas D, Hasson D, Liu CY, Amar AP. The importance of preoperative diagnosis of blister aneurysms. J Clin Neurosci. 2015. 22: 1408-12
28. Shah SS, Gersey ZC, Nuh M, Ghonim HT, Elhammady MS, Peterson EC. Microsurgical versus endovascular interventions for blood-blister aneurysms of the internal carotid artery: Systematic review of literature and meta-analysis on safety and efficacy. J Neurosurg. 2017. 127: 1361-73
29. Walsh KM, Moskowitz SI, Hui FK, Spiotta AM. Multiple overlapping stents as monotherapy in the treatment of “blister” pseudoaneurysms arising from the supraclinoid internal carotid artery: A single institution series and review of the literature. J Neurointerv Surg. 2014. 6: 184-94
30. Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man. J Neurosurg. 1978. 48: 173-8