- Department of Diagnostic and Interventional Radiology, University Hospital Center, Osijek, Croatia.
- Faculty of Medicine, Josip Juraj Strossmayer University of Osijek, Croatia.
- Department of Maxillofacial and Oral Surgery, University Hospital Center, Osijek, Croatia.
- Department of Neurosurgery, University Hospital Center, Osijek, Croatia.
- Department of Neurology, University Hospital Center, Osijek, Croatia.
- Department of Information Sciences, Faculty of Humanities and Social Sciences, Josip Juraj Strossmayer University of Osijek, Osijek, Croatia.
Correspondence Address:
Nenad Koruga, Department of Neurosurgery, University Hospital Center Osijek, 31000 Osijek, Croatia.
DOI:10.25259/SNI_906_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Vjekoslav Kopačin1,2, Vedran Zubčić2,3, Ivan Mumlek2,3, Dario Mužević2,4, Alen Rončević2,4, Ana-Maria Lazar2,3, Ana Kvolik Pavić2,3, Anamarija Soldo Koruga2,5, Zdravka Krivdić1,2, Ivana Martinović6, Nenad Koruga2,4. Personalized 3D-printed cranial implants for complex cranioplasty using open-source software. 09-Feb-2024;15:39
How to cite this URL: Vjekoslav Kopačin1,2, Vedran Zubčić2,3, Ivan Mumlek2,3, Dario Mužević2,4, Alen Rončević2,4, Ana-Maria Lazar2,3, Ana Kvolik Pavić2,3, Anamarija Soldo Koruga2,5, Zdravka Krivdić1,2, Ivana Martinović6, Nenad Koruga2,4. Personalized 3D-printed cranial implants for complex cranioplasty using open-source software. 09-Feb-2024;15:39. Available from: https://surgicalneurologyint.com/surgicalint-articles/12736/
Abstract
Background: Cranioplasty is a routine neurosurgery treatment used to correct cranial vault abnormalities. Utilization of 3D printing technology in the field of cranioplasty involving the reconstruction of cranial defects emerged as an advanced possibility of anatomical reshaping. The transformative impact of patient-specific 3D printed implants, focuses on their remarkable accuracy, customization capabilities, and enhanced biocompatibility.
Methods: The precise adaptation of implants to patient-specific anatomies, even in complex cases we presented, result in improved aesthetic outcomes and reduced surgical complications. The ability to create highly customized implants addresses the functional aspects of cranial defects and considers the psychological impact on patients.
Results: By combining technological innovation with personalized patient care, 3D printed cranioplasty emerges as a transformative avenue in cranial reconstruction, ultimately redefining the standards of success in neurosurgery.
Conclusion: 3D printing allows an excellent cranioplasty cosmesis achieved at a reasonable price without sacrificing patient outcomes. Wider implementation of this strategy can lead to significant healthcare cost savings.
Keywords: 3D Printing, Cranioplasty, Implant, Open-source, Patient care, Personalized medicine, Polymethyl methacrylate, Precision medicine, Printing, Prosthetic, Three-dimensional
INTRODUCTION
The neurocranium acts as a barrier between the outer world and the brain. Without its support, even a little head injury could result in harm to the brain parenchyma, which could lead to impairment or even death. Neurosurgeons must restore the bone vault’s functional and cosmetic qualities if it is damaged by trauma, tumors, infection, or iatrogenic causes. Since Fallopius employed a gold plate to replace a bone deficiency in the 16th century, cranioplasty as a surgical operation has been known and practiced for a very long time.[
MATERIALS AND METHODS
DICOM data acquisition
A patient in whom the use of any advanced visualization methods is planned should certainly have one of the available three-dimensional diagnostic imaging methods performed. Due to a good bone tissue depiction, in CMF reconstruction surgery, the most commonly used imaging method is computed tomography (CT), followed by magnetic resonance imaging (MRI), where different soft tissues have excellent contrast to each other.[
How we do it: If the affected region contains more anatomical details, thin layers (0.625 mm) are reconstructed, but usually the thickness of the layers does not exceed 1 mm. In the case of tumor changes, the application of intravenous contrast is mandatory using an automated contrast injector (contrast volume and flow depending on the case). Soft tissue and bone reconstructions are sent to picture archiving and communication systems.
Computer-aided design (CAD) 3D model generation and 3D printing of the model for preoperative planning
The process of virtual surgical planning and virtual surgery, personalized surgical guides, and implant fabrication starts with the segmentation of the desired anatomy from obtained DICOM data and the generation of CAD 3D models. For the segmentation and 3D model generation, numerous free and open-source software are available.[
How we do it: For the segmentation, the authors prefer 3D Slicer, an open-source software for visualization, segmentation, registration, and analysis of medical, biomedical, and other 3D images and meshes, as well as for planning and navigating image-guided procedures.[
Each model is appropriately labeled by adding the patient’s name, surname, and year of birth on the opposite side of the visible existing pathology on the model.
The standard tessellation language (STL) file of the model is imported in slicer software where a.gcode file is being created containing previously defined parameters such as material for 3D printing being used and layer height.
FDM/FFF technology-based 3D printer, Prusa i3 MK3 (Prusa Research, Prague, Czech Republic), is used for 3D printing of the haptic 3D model used for preoperative planning. Polylactic acid (PLA) filament, being the most available, most affordable, and easiest for 3D printing, is used for the generation of such anatomical 3D models.
Preoperative planning/virtual surgery
Several studies that have been published show the advantages of using advanced visualization, such as extended reality and 3D printing, in preoperative planning and understanding three-dimensional anatomy compared to conventional imaging (CT or MRI).[
How we do it
A multidisciplinary team consisting of maxillo-facial, neurosurgeons, and radiologists is assembled when the patient requires some cranial base surgery and reconstruction is being presented. A haptic, 3D-printed model of pathologically altered anatomy and a CAD 3D model are being presented to the team. Together, they decide on the osteotomy margins.
3D modeling and 3D printing of the surgical guides and matching implant molds
The majority of surgical cutting guides or personalized implant production remains within the realm of commercial biomechanical engineering companies, necessitating outsourcing and incurring significant costs. Although commercial software options exist, they typically come with a higher price.
European Union (EU) Regulations on medical devices (MDR 2017/745, item 30) state that it is possible to use open-source solutions for in-house and non-industrial scale production of medical devices, that is, surgical cutting guides and implants. Therefore, it is no wonder that clinicians in the EU, as well as others around the world, already widely accept such free solutions in CMF surgeries.[
How we do it
We concluded that the most affordable and equally effective method for us is the 3D printing of surgical guides and molds into which PMMA will be injected. A detailed presentation and video tutorial can be found in the link given in the Supplementary Material.
There are a number of free solutions available for the 3D modeling process, and it is best to use the ones you are most familiar with. The authors prefer open-source 3D modeling software Blender and Meshmixer—first, the.STL file of the 3D model is imported into the Blender. If the file is large, the decimate function on the 3D model is performed, or a part of the unnecessary anatomy is deleted to reduce the number of facets and thereby speed up the computing power of the computer and 3D modeling process. In case there is significant bone destruction and the bone is missing at the site of the planned cranioplasty using a personalized implant, mirroring of the healthy, contralateral half of the skull can be done. Suppose there is a diagnostic imaging study performed before pronounced bone destruction. In that case, it is possible to make and import a 3D model of healthy anatomy that is later used in the design of the implant, or it is possible to manually model and close the bone defect, obtaining a “healthy,” unaffected region.
According to the preoperative plan, the width between the destroyed bone and the surgical guides is usually around 10 mm to ensure a healthy margin. The width of the cutting guide must be sufficient to contain the hole for the fixing screws (the diameter depends on the manufacturer. Usually it is 3 mm) and at least 2–3 mm from the edge so that it does not break – usually 8–10 mm is enough. In places where there is extremely little space for manipulation, such as the orbit, it is possible to design narrow guides, and then fixation screw holes are not placed on these segments.
It is preferable to design the guides so that the osteotomies are not perpendicular to the calvaria but that the corresponding implant sits like a wedge in place, thus ensuring a larger contact surface with the bone and reducing the load on the osteosynthetic material. At the base of the skull, due to the geometry, this will often not be feasible, and then the angle of the osteotomy can be perpendicular to the bone.
After the surgical guides design and according to the osteotomy plane, the next step is the generation of the implant and the two-part mold for injection.
Although there are some publications where the use of polycaprolactone and PLA in the production of personalized medical devices is mentioned, the authors believe that more studies with a longer follow-up time are needed to confirm their safe use in personalized implant fabrication, so we do not use them regularly.[
After the 3D printing, surgical guides and molds were sterilized in a low-temperature sterilizer with hydrogen peroxide plasma. Implants are prepared during the surgery.
RESULTS
Preoperative planning
All patients were thoroughly clinically and radiologically evaluated according to the need for cranial surgery and the possible use of 3D-printed molds. Preoperative 3D-printed anatomical models were used for more precise craniotomy to spare healthy anatomical borders and to reduce bleeding and the timespan of surgery [
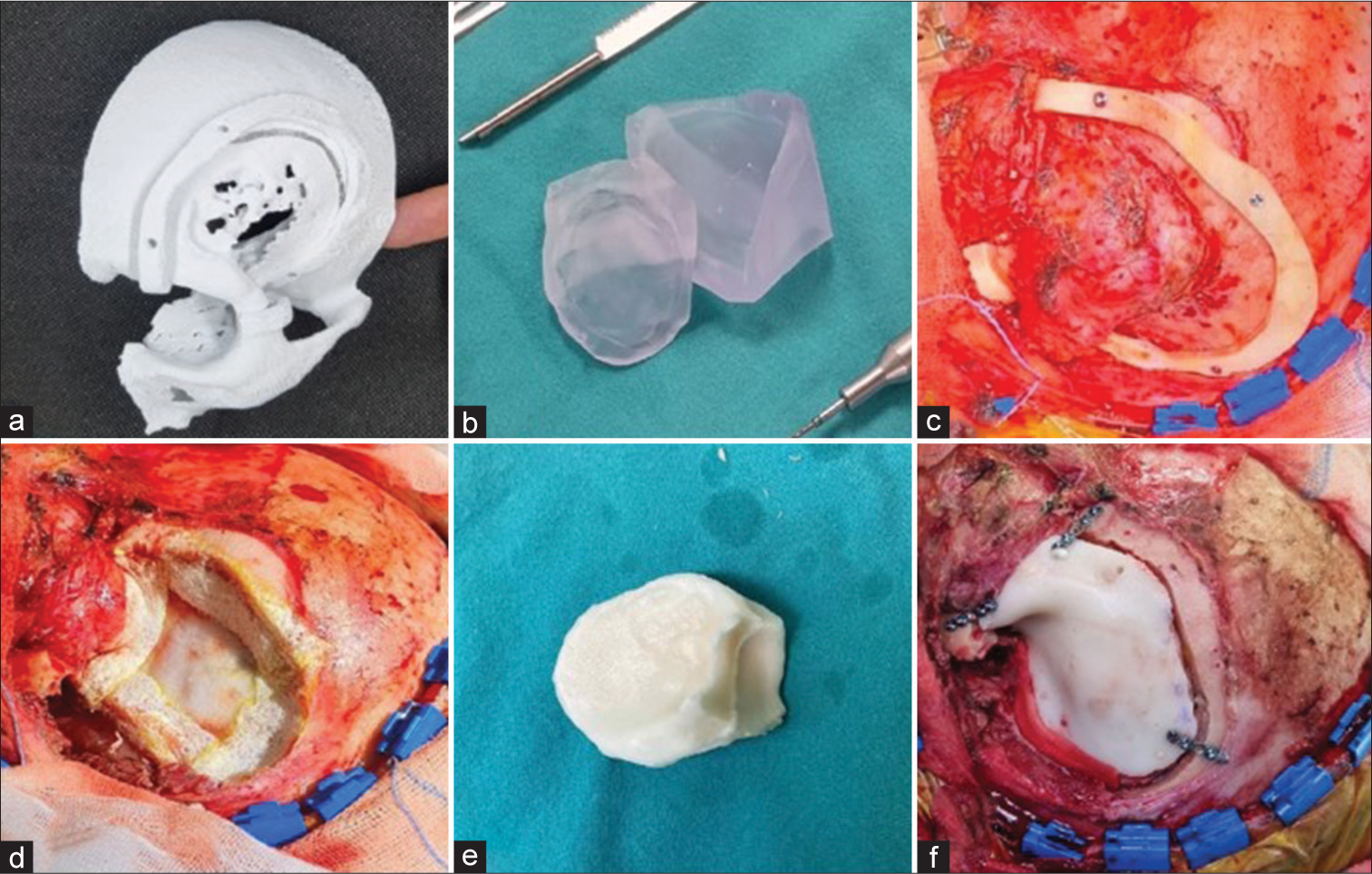
Figure 1:
(a) Preoperatively 3D printed model of the pathologically altered anatomy and concomitant surgical guide, (b) 3D printed molds, (c) 3D printed surgical guide fixed with the screws in order to achieve appropriate craniotomy, (d) the surgical site completely prepared for a prosthesis, (e) Polymethyl-meth-acrylate (PMMA) prosthesis ready for the fixation, (f) operculum fixed with mini-screws, surgical site anatomically reshaped.
Surgical technique
All patients underwent surgery in general anesthesia in a regular fashion and surgeries were done multidisciplinary. Furthermore, all patients underwent craniotomies at tumor sites according to preoperatively planned approaches. On the operative day, the molds were pretreated, sterilized placed in plastic bags. The PMMA components were mixed, and the dough-like PMMA mixture was placed between the two mold parts, which were then clamped together. The molds and PMMA are placed in a cold saline solution during polymerization. Excess PMMA material exits the mold through the installed chimney holes in the molds, and it was removed after the polymerization. Edges of polymerized PMMA were cautiously examined and were additionally drilled or removed by the Luer pliers if necessary. After the implant was completed and ready, it was repeatedly sterilized in the alcoholic solution. Care was implemented to avoid contact with any non-sterilized surfaces before implantation. Operculum was then implanted at the site of craniotomy and fixed with mini-plates and screws (Stryker cranial fixation system, Stryker, MI USA) [
Case 1
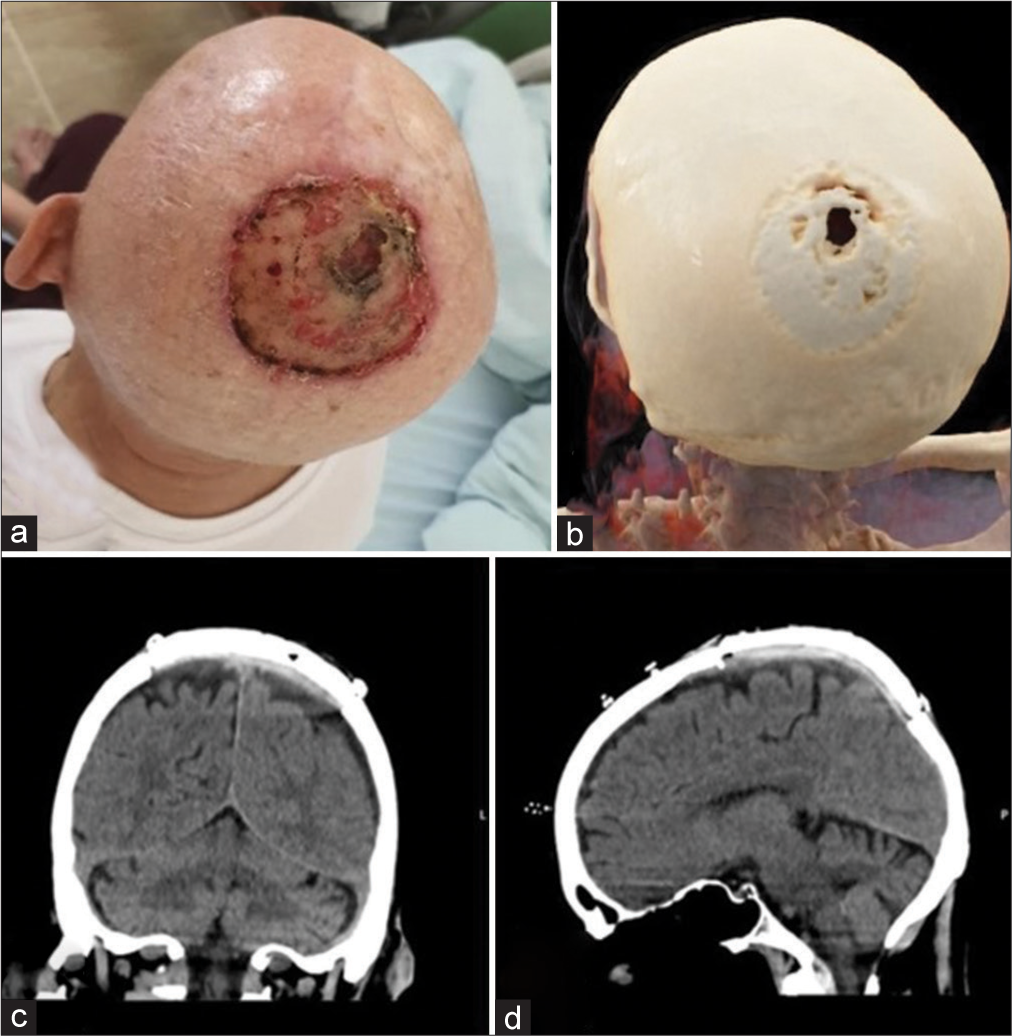
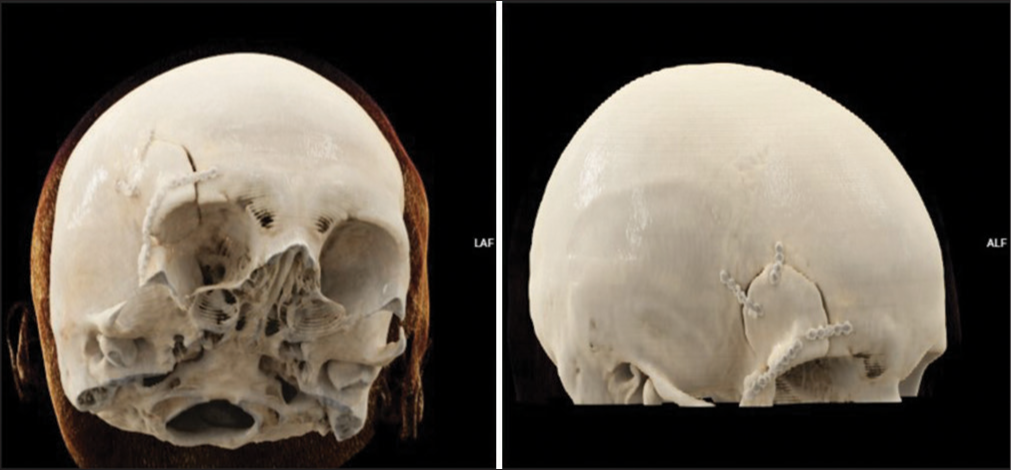
A maxillofacial surgeon primarily treated a 75-year-old male patient due to an aggressive calvarial tumor. The patient neglected aggressive carcinoma, which pierced the parietal bone and skin of the scalp, confirmed by a CT scan [
Figure 2:
(a) Preoperative finding of planocellular carcinoma with continuous spread through the calvarial skin and parietal bone, (b) Cinematic rendering (CRT) of the head CT scan showing bone destruction, (c) coronal and (d) sagittal plane of the postoperative, non-enhanced CT scan one week after the surgery revealed an excellent implant fixation.
Case 2
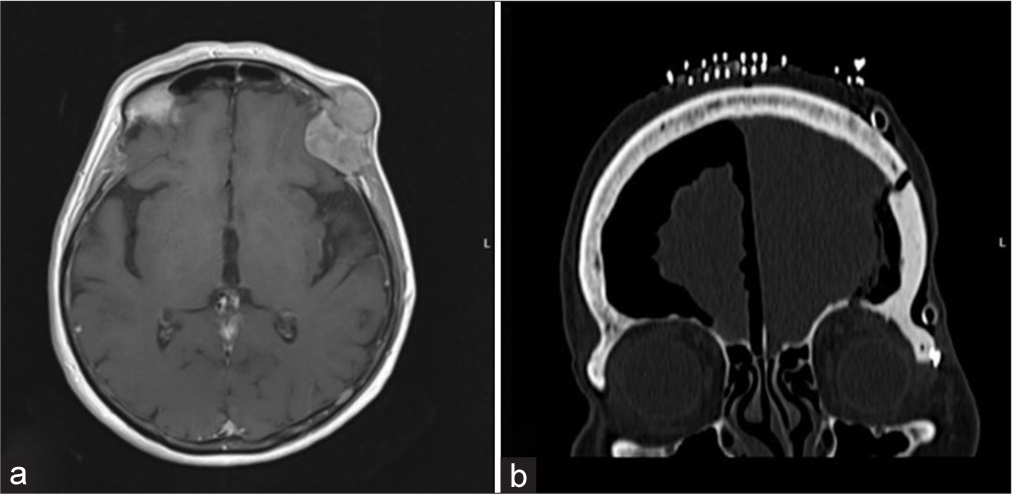
An 80-year-old female patient noticed an unusual convexed hard consistency process on the left-sided orbit. MRI scanning confirmed tumoral tissue which occupied the frontal bone and spreaded extracranially as well as intracraniallyextradurally [
Case 3
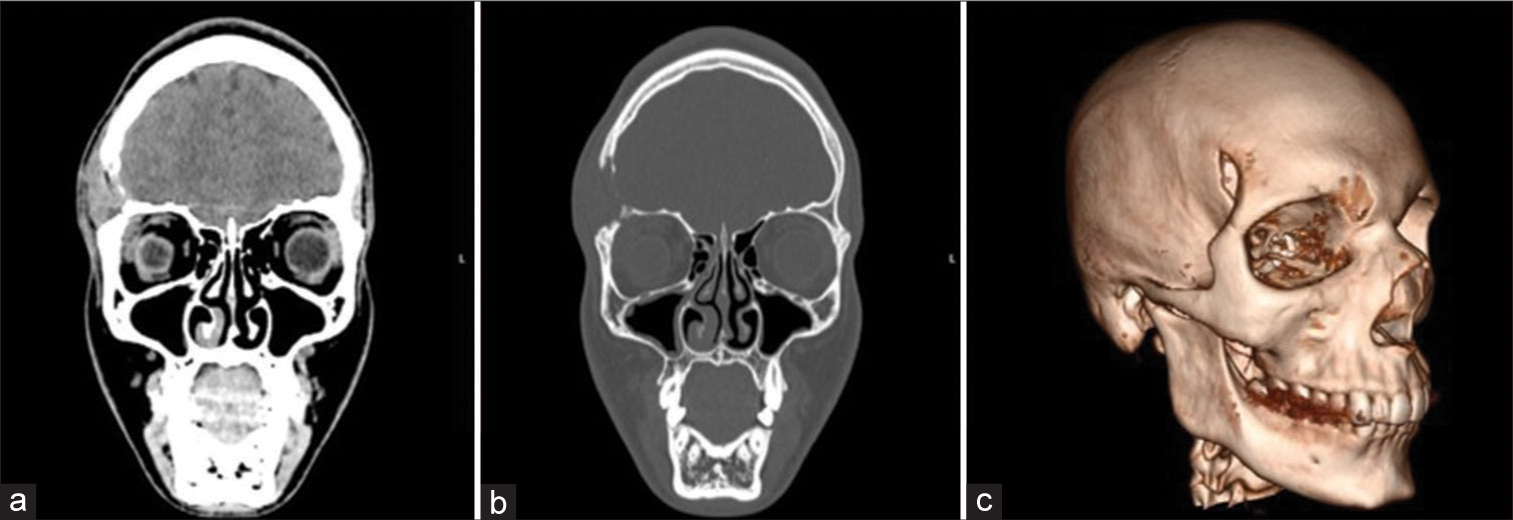
A 17-year-old boy noticed an unusual edema painful after finger compression at the site on the right-sided orbit [Figure 4ac]. Preoperative cytologic biopsy pointed at eosinophilic granuloma, which was later confirmed postoperatively by pathohistological verification. The patient underwent surgery in a general fashion after a bi-coronal approach. Intra- and postoperative periods went uneventfully, and the postoperative CT scan revealed an excellent position of the operculum [
DISCUSSION
In terms of preserving esthetically attractive results, the human skull is one of the most complicated parts of the body. The creation of individualized endoprostheses by cranioplasty is necessary in the case of a posttraumatic or postoperative defect to give affected individuals a respectable quality of life.[
However, compared to implants created using preoperative CT scan-based 3D printing, freehand PMMA sculpting has generally produced worse results in terms of both material and technique. These outcomes include a higher prevalence of wound healing disorders, higher rates of early re-operative revisions, a higher prevalence of extradural hematoma, CSF leak, poor radiological accuracy, and inferior cosmetic results. Using a home 3D printer with the absolute minimum requirements and free software, it is possible to benefit from PMMA’s affordability while improving its cosmetic accuracy, with the minimum amount of difficulty.[
CONCLUSION
Cranioplasty is a common surgical procedure widely accepted and performed all over the world. The advantages of its use include a simple and fast process of production, availability of materials, and excellent cosmetic effects. Nevertheless, some disadvantages emerged, such as the selection of the best suitable producing materials as well as its price. The use of the most appropriate, biocompatible, strong, and durable materials remains the main goal of its production. The possibility of homemade production of 3D-printed implants might play a significant role in low-income countries, although in some cases, its price is immensely increased. We have to emphasize that despite some shortcomings and disadvantages, the use of 3D-printed implants remains the best feasible option in everyday use in terms of time sparing, anatomical accuracy, and consequent cosmetic effects.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY FILE
Supplementary Material: Video tutorial:
References
1. Alkhaibary A, Alharbi A, Alnefaie N, Almubarak AO, Aloraidi A, Khairy S. Cranioplasty: A comprehensive review of the history, materials, surgical aspects, and complications. World Neurosurg. 2020. 139: 445-52
2. Arnaoutakis D, Bahrami A, Cohn JE, Smith JE. Cranioplasty using a mixture of biologic and nonbiologic agents. JAMA Facial Plast Surg. 2018. 20: 9-13
3. Ashraf M, Choudhary N, Kamboh UA, Raza MA, Sultan KA, Ghulam N. Early experience with patient-specific low-cost 3D-printed polymethylmethacrylate cranioplasty implants in a lower-middle-income-country: Technical note and economic analysis. Surg Neurol Int. 2022. 13: 270
4. Bonda DJ, Manjila S, Selman WR, Dean D. The recent revolution in the design and manufacture of cranial implants: Modern advancements and future directions. Neurosurgery. 2015. 77: 814-24
5. Bowers CA, Riva-Cambrin JA, Hertzler DA, Walker ML. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr. 2013. 11: 526-32
6. Codubix proteza kości czaszki niejałowa. Available from: https://www.matopat24.pl/codubix-proteza-kosci-czaszkiniejalowa_7341-14736 [Last accessed on 2022 Jun 19].
7. Csámer L, Csernátony Z, Novák L, Kővári VZ, Kovács ÁÉ, Soósné Horváth H. Custom-made 3D printing-based cranioplasty using a silicone mould and PMMA. Sci Rep. 2023. 13: 11985
8. Czyżewski W, Jachimczyk J, Hoffman Z, Szymoniuk M, Litak J, Maciejewski M. Low-cost cranioplasty-a systematic review of 3D printing in medicine. Materials (Basel). 2022. 15: 4731
9. Egger J, Gall M, Tax A, Ücal M, Zefferer U, Li X. Interactive reconstructions of cranial 3d implants under MeVisLab as an alternative to commercial planning software. PLoS One. 2017. 12: e0172694
10. Evins AI, Dutton J, Imam SS, Dadi AO, Xu T, Cheng D. On-demand intraoperative 3-dimensional printing of custom cranioplastic prostheses. Oper Neurosurg (Hagerstown). 2018. 15: 341-9
11. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S. 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012. 30: 1323-41
12. Ganau M, Cebula H, Fricia M, Zaed I, Todeschi J, Scibilia A. Surgical preference regarding different materials for custom-made allograft cranioplasty in patients with calvarial defects: Results from an internal audit covering the last 20 years. J Clin Neurosci. 2020. 74: 98-103
13. Garg R, Aggarwal A, Salunke P. Importance of calvaria in cerebrospinal fluid dynamics: A case of ventriculomegaly and sinking flap syndrome after decompressive craniectomy. Asian J Neurosurg. 2018. 13: 128-9
14. Goldstein JA, Paliga JT, Bartlett SP. Cranioplasty: Indications and advances. Curr Opin Otolaryngol Head Neck Surg. 2013. 21: 400-9
15. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9
16. Hurson C, Tansey A, O’Donnchadha B, Nicholson P, Rice J, McElwain J. Rapid prototyping in the assessment, classification and preoperative planning of acetabular fractures. Injury. 2007. 38: 1158-62
17. Iaccarino C, Kolias A, Roumy LG, Fountas K, Adeleye AO. Cranioplasty following decompressive craniectomy. Front Neurol. 2020. 10: 1357
18. Kim MJ, Lee HB, Ha SK, Lim DJ, Kim SD. Predictive factors of surgical site infection following cranioplasty: A study including 3D printed implants. Front Neurol. 2021. 12: 745575
19. Las DE, Verwilghen D, Mommaerts MY. A systematic review of cranioplasty material toxicity in human subjects. J Craniomaxillofac Surg. 2021. 49: 34-46
20. Leão RS, Maior JR, Lemos CA, Vasconcelos BC, Montes MA, Pellizzer EP. Complications with PMMA compared with other materials used in cranioplasty: A systematic review and meta-analysis. Braz Oral Res. 2018. 32: e31
21. Lönnemark O, Ryttlefors M, Sundblom J. Cranioplasty in brain tumor surgery : A single-center retrospective study investigating cranioplasty failure and tumor recurrence. World Neurosurg. 2023. 170: e313-23
22. Malcolm JG, Mahmooth Z, Rindler RS, Allen JW, Grossberg JA, Pradilla G. Autologous cranioplasty is associated with increased reoperation rate: A systematic review and meta-analysis. World Neurosurg. 2018. 116: 60-8
23. Malcolm JG, Rindler RS, Chu JK, Grossberg JA, Pradilla G, Ahmad FU. Complications following cranioplasty and relationship to timing: A systematic review and meta-analysis. J Clin Neurosci. 2016. 33: 39-51
24. Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien). 2006. 148: 535-40
25. Mitsouras D, Liacouras P, Imanzadeh A, Giannopoulos AA, Cai T, Kumamaru KK. Medical 3D printing for the radiologist. Radiographics. 2015. 35: 1965-88
26. Moore EJ, Price DL, Van Abel KM, Janus JR, Moore ET, Martin E. Association of virtual surgical planning with external incisions in complex maxillectomy reconstruction. JAMA Otolaryngol Head Neck Surg. 2021. 147: 526-31
27. Muff JL, Heye T, Thieringer FM, Brantner P. Clinical acceptance of advanced visualization methods: A comparison study of 3D-print, virtual reality glasses, and 3D-display. 3D Print Med. 2022. 8: 5
28. Ostaș D, Almășan O, Ileșan RR, Andrei V, Thieringer FM, Hedeșiu M. Point-of-care virtual surgical planning and 3D printing in oral and cranio-maxillofacial surgery: A narrative review. J Clin Med. 2022. 11: 6625
29. Pöppe JP, Spendel M, Schwartz C, Winkler PA, Wittig J. The “Springform” technique in cranioplasty: Custom made 3D-printed templates for intraoperative modelling of polymethylmethacrylate cranial implants. Acta Neurochir (Wien). 2022. 164: 679-88
30. Poukens J, Haex J, Riediger D. The use of rapid prototyping in the preoperative planning of distraction osteogenesis of the craniomaxillofacial skeleton. Comput Aided Surg. 2003. 8: 146-54
31. Qin L, Yao S, Zhao J, Zhou C, Oates T, Weir M. Review on development and dental applications of polyetheretherketone-based biomaterials and restorations. Materials (Basel). 2021. 14: 408
32. Ritschl LM, Kilbertus P, Grill FD, Schwarz M, Weitz J, Nieberler M. In-house, open-source 3d-software-based, CAD/CAM-planned mandibular reconstructions in 20 consecutive free fibula flap cases: An explorative cross-sectional study with three-dimensional performance analysis. Front Oncol. 2021. 11: 731336
33. Rybicki FJ, Grant GT, editors. 3D printing in medicine: A practical guide for medical professionals. Germany: Springer; 2017. p.
34. Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev. 2019. 12: CD003983
35. Sanan A, Haines SJ. Repairing holes in the head: A history of cranioplasty. Neurosurgery. 1997. 40: 588-603
36. Satapathy D, Nadeem M, Shukla DP, Prabhuraj A, Devi BI. Cosmetic outcome of cranioplasty after decompressive craniectomy-an overlooked aspect. World Neurosurg. 2019. 129: e81-6
37. Sharma N, Aghlmandi S, Dalcanale F, Seiler D, Zeilhofer HF, Honigmann P. Quantitative assessment of point-of-care 3D-printed patient-specific polyetheretherketone (PEEK) cranial implants. Int J Mol Sci. 2021. 22: 8521
38. Shih FY, Lin CC, Wang HC, Ho JT, Lin CH, Lu YT. Risk factors for seizures after cranioplasty. Seizure. 2019. 66: 15-21
39. Šimić L, Kopačin V, Mumlek I, Butković J, Zubčić V. Improved technique of personalised surgical guides generation for mandibular free flap reconstruction using an open-source tool. Eur Radiol Exp. 2021. 5: 30
40. Stephens FL, Mossop CM, Bell RS, Tigno T, Rosner MK, Kumar A. Cranioplasty complications following wartime decompressive craniectomy. Neurosurg Focus. 2010. 28: E3
41. Tan ET, Ling JM, Dinesh SK. The feasibility of producing patient-specific acrylic cranioplasty implants with a low-cost 3D printer. J Neurosurg. 2016. 124: 1531-7
42. van de Vijfeijken SE, Münker TJ, Spijker R, Karssemakers LH, Vandertop WP, Becking AG. Autologous bone is inferior to alloplastic cranioplasties: Safety of autograft and allograft materials for cranioplasties, a systematic review. World Neurosurg. 2018. 117: 443-52.e8
43. von Steinbuechel N, Wilson L, Gibbons H, Hawthorne G, Höfer S, Schmidt S. Quality of life after brain injury (QOLIBRI): Scale validity and correlates of quality of life. J Neurotrauma. 2010. 27: 1157-65
44. Wang P, Que W, Zhang M, Dai X, Yu K, Wang C. Application of 3-dimensional printing in pediatric living donor liver transplantation: A single-center experience. Liver Transpl. 2019. 25: 831-40
45. Worm PV, Finger G, do Nascimento TL, Rynkowski CB, Collares MV. The impact of cranioplasty on the patients’ quality of life. J Craniomaxillofac Surg. 2019. 47: 715-9
46. Yue JK, Rick JW, Deng H, Feldman MJ, Winkler EA. Efficacy of decompressive craniectomy in the management of intracranial pressure in severe traumatic brain injury. J Neurosurg Sci. 2019. 63: 425-40