- Professor of Clinical Neurosurgery, School of Medicine, State University of NY at Stony Brook and Editor-in-Chief Surgical Neurology International NY, USA, and c/o Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY, USA.
Correspondence Address:
Nancy E. Epstein, M.D., F.A.C.S., Professor of Clinical Neurosurgery, School of Medicine, State University of NY at Stony Brook, and Editor-in-Chief of Surgical Neurology International NY, USA, and c/o Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY, USA.
DOI:10.25259/SNI_119_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Perspective: How can risks to patients be limited during spine surgeons’ learning curves?. 22-Mar-2024;15:97
How to cite this URL: Nancy E. Epstein. Perspective: How can risks to patients be limited during spine surgeons’ learning curves?. 22-Mar-2024;15:97. Available from: https://surgicalneurologyint.com/surgicalint-articles/12817/
Abstract
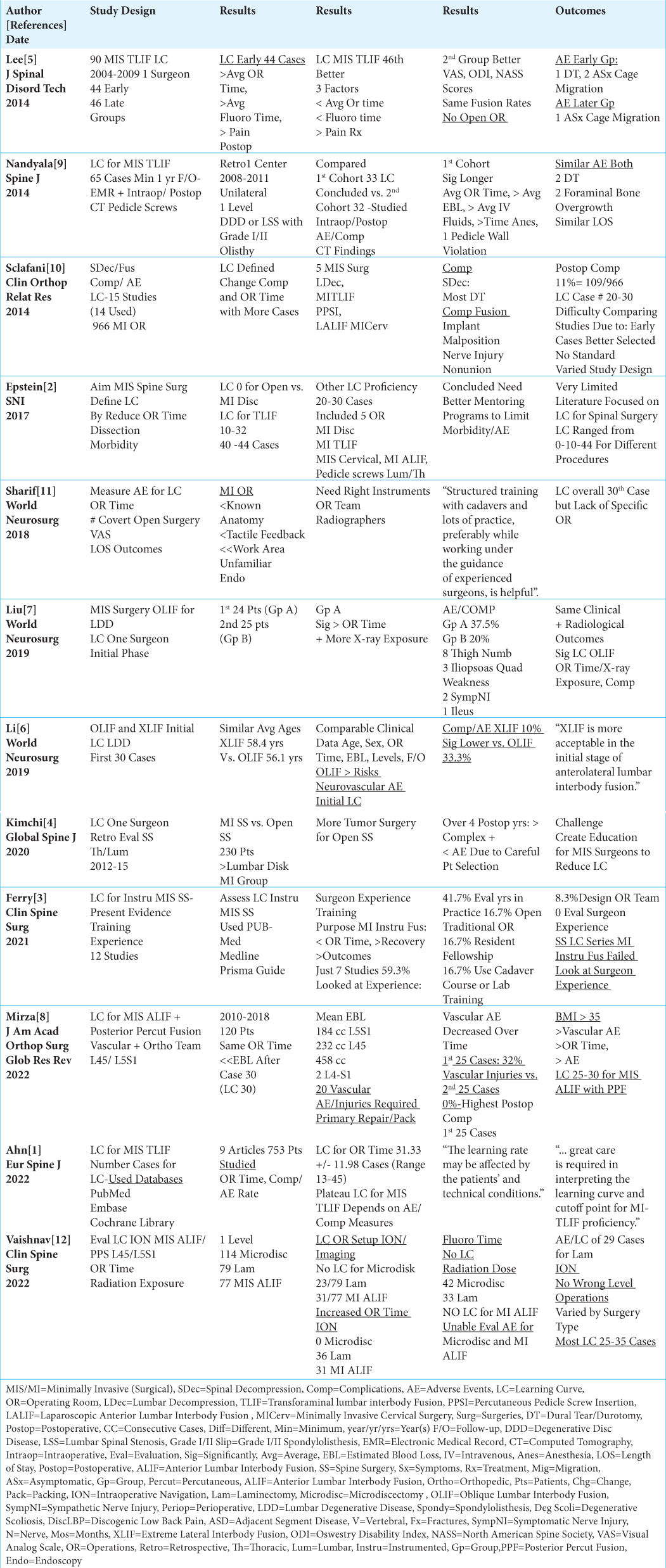
Background: Learning curves (LC) are typically defined by the number of different spinal procedures surgeons must perform before becoming “proficient,” as demonstrated by reductions in operative times, estimated blood loss (EBL), length of hospital stay (LOS), adverse events (AE), fewer conversions to open procedures, along with improved outcomes. Reviewing 12 studies revealed LC varied widely from 10-44 cases for open vs. minimally invasive (MI) lumbar diskectomy, laminectomy, transforaminal lumbar interbody fusion (TLIF), anterior lumbar interbody fusion (ALIF), and oblique/extreme lateral interbody fusions (OLIF/XLIF). We asked whether the risks of harm occurring during these LC could be limited if surgeons routinely utilized in-person/intraoperative mentoring (i.e., via industry, academia, or well-trained colleagues).
Methods: We evaluated LC for multiple lumbar operations in 12 studies.
Results: These studies revealed no LC for open vs. MI lumbar diskectomy. LC required 29 cases for MI laminectomy, 10-44 cases for MI TLIF, 24-30 cases for MI OLIF, and 30 cases for XLIF. Additionally, the LC for MI ALIF was 30 cases; one study showed that 32% of major vascular injuries occurred in the first 25 vs. 0% for the next 25 cases. Shouldn’t the risks of harm to patients occurring during these LC be limited if surgeons routinely utilized in-person/intraoperative mentoring?
Conclusions: Twelve studies showed that the LC for at different MI lumbar spine operations varied markedly (i.e., 10-44 cases). Wouldn’t and shouldn’t spine surgeons avail themselves of routine in-person/intraoperative mentoring to limit patients’ risks of injury during their respective LC for these varied spine procedures ?
Keywords: Learning Curve, Risks, Adverse Events, Neurological Deficits, Pros, Cons, Morbidity, Minimally Invasive (MI), Transforaminal Lumbar Interbody Fusions (TLIF), Anterior Lumbar Interbody Fusion (ALIF), Oblique/Extreme Lateral Interbody Fusions (OLIF/XLIF), Other Operations, Outcomes, Definition Learning Curve, Need for Mentoring
INTRODUCTION
For spinal surgeons, learning curves (LC) are defined by the number of spine procedures surgeons must perform before becoming “proficient” as demonstrated by reductions in operative times, estimated blood loss (EBL), length of hospital stays (LOS), adverse events (AE), fewer conversions to open procedures, and with improved outcomes. During neurosurgery or orthopedic residency training programs, the risks to patients during residents’ learning curves (LC) are limited by the attending surgeons’ “direct supervision”. However, how are these risks mitigated during the varied LC documented for new/different spine procedures introduced after residency (i.e., minimally invasive (MI) diskectomy, laminectomy, transforaminal lumbar interbody fusion (TLIF), anterior lumbar interbody fusion (ALIF), and oblique/extreme lateral interbody fusion (OLIF/XLIF), cervical fusions) [
Defining Learning Curves for Minimally Invasive (MI) Spine Operations
Minimally invasive (MI) spine operations were largely devised to reduce operative time, tissue trauma, and perioperative morbidity [
No Learning Curve for Microdiskectomy
Several studies documented no LC cases were required for conversion from open to performing MI microdiscectomy [
Learning Curve for MI Transforaminal Lumbar Interbody Fusion (TLIF)
Six studies focused on the wide variation in LC reported for MI TLIF [
LC for MI Oblique Lumbar Interbody Fusion (OLIF) and Extreme Lateral Interbody Fusion (XLIF)
Two studies showed the LC for OLIF was achieved after 24-30 cases vs. 30 cases required to satisfy the LC for XLIF [
LC for MI Anterior Lumbar Interbody Fusions (ALIF)
Mirza et al. (2022) found the LC included the first 25 - 30 cases out of 120 MI ALIF performed with posterior percutaneous instrumentation at the L45 and L5S1 levels (2010-2018) [
Satisfied with In-Person/Intraoperative Mentoring Provided by Manufacturers, Academia, or Well-Trained Colleagues Could Limit the LC for MI Spine Surgery
Although some companies/manufacturers of spinal instrumentation provide “mentors” to directly scrub/supervise spine surgeons performing new operations, how many spine surgeons request and/or receive this “help”? Most likely, inexperienced spine surgeons return home and begin performing these procedures. Typically, they don’t consult experts or well-trained colleagues at surrounding academic/non-academic institutions, particularly if they are in competing groups or specialties (i.e., neurosurgery vs. orthopedics) or at surrounding institutions.
Risks and Remediation of Spinal Surgeons’ Learning Curves for MI Spine Operations
Several authors focused on the risks to patients during spine surgeons’ LC for different MI spine procedures, and potential remediation maneuvers [
CONCLUSION
Twelve studies showed that the LC for different MI lumbar spine operations varied markedly (i.e., 10-44 cases) [
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ahn Y, Lee S, Kim WK, Lee SG. Learning curve for minimally invasive transforaminal lumbar interbody fusion: A systematic review. Eur Spine J. 2022. 31: 3551-9
2. Epstein NE. Learning curves for minimally invasive spine surgeries: Are they worth it?. Surg Neurol Int. 2017. 8: 61
3. Ferry C. Characterizing the surgeon learning curve in instrumented minimally invasive spinal surgery: Does the evidence account for training and experience? A systematic literature review. Clin Spine Surg. 2021. 34: 17-21
4. Kimchi G, Orlev A, Hadanny A, Knoller N, Harel R. Minimally invasive spine surgery: The learning curve of a single surgeon. Global Spine J. 2020. 10: 1022-6
5. Lee KH, Yeo W, Soeharno H, Yue WM. Learning curve of a complex surgical technique: Minimally invasive transforaminal lumbar interbody fusion (MIS TLIF). J Spinal Disord Tech. 2014. 27: E234-40
6. Li J, Wang X, Sun Y, Shang F. Gao Y, Li Z. Safety analysis of two anterior lateral lumbar interbody fusions at the initial stage of learning curve. World Neurosurg. 2019. 127: e901-9
7. Liu C, Wang J, Zhou Y. Perioperative complications associated with minimally invasive surgery of oblique lumbar interbody fusions for degenerative lumbar diseases in 113 patients. Clin Neurol Neurosurg. 2019. 184: 105381
8. Mirza MZ, Olson SL, Panthofer AM, Matsumura JS, Williams SK. Surgeon learning curve and clinical outcomes of minimally invasive anterior lumbar interbody fusion with posterior percutaneous instrumentation. J Am Acad Orthop Surg Glob Res Rev. 2022. 6: e22.00207
9. Nandyala SV, Fineberg SJ, Pelton M, Singh K. Minimally invasive transforaminal lumbar interbody fusion: One surgeon’s learning curve. Spine J. 2014. 14: 1460-5
10. Sclafani JA, Kim CW. Complications associated with the initial learning curve of minimally invasive spine surgery: A systematic review. Clin Orthop Relat Res. 2014. 472: 1711-7
11. Sharif S, Afsar A. Learning curve and minimally invasive spine surgery. World Neurosurg. 2018. 119: 472-8
12. Vaishnav AS, Gang CH, Qureshi SA. Time-demand, radiation exposure and outcomes of minimally invasive spine surgery with the use of skin-anchored intraoperative navigation: The effect of the learning curve. Clin Spine Surg. 2022. 35: E111-20