- Professor of Clinical Neurosurgery, School of Medicine, State University of NY at Stony Brook and Editor-in-Chief Surgical Neurology International NY, USA, and c/o Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY, USA,
- Assistant Clinical Professor of Orthopedics, NYU Langone Hospital, Long Island, NY, USA, 1122 Frankling Avenue Suite 106, Garden City, NY, USA.
Correspondence Address:
Nancy E Epstein, M.D., F.A.C.S. Professor of Clinical Neurosurgery, School of Medicine, State University of NY at Stony Brook and Editor-in-Chief Surgical Neurology International NY, USA, and c/o Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY, USA.
DOI:10.25259/SNI_772_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein1, Marc A Agulnick2. Perspective: Postoperative spinal epidural hematomas (pSEH) should be treated, not ignored. 13-Oct-2023;14:363
How to cite this URL: Nancy E. Epstein1, Marc A Agulnick2. Perspective: Postoperative spinal epidural hematomas (pSEH) should be treated, not ignored. 13-Oct-2023;14:363. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12594
Abstract
Background: Patients with postoperative spinal epidural hematomas (pSEH) typically require emergency treatment to avoid paralysis; these hematomas should not be ignored. pSEH patients need to undergo immediate MR studies to document the location/extent of their hematomas, and emergent surgical decompression with/ without fusion if warranted.
Methods: The frequencies of symptomatic pSEH ranged in various series from 0.1%-4.46%. Major predisposing factors included; perioperative/postoperative coagulation abnormalities/disorders, multilevel spine surgeries, previous spine surgery, and intraoperative cerebrospinal fluid (CSF) leaks. For surgery at all spinal levels, one study observed pSEH developed within an average of 2.7 postoperative hours. Another series found 100% of cervical/thoracic, and 50% of lumbar pSEH were symptomatic within 24 postoperative hrs., while a third series noted a 24-48 postoperative window for pSEH to develop.
Results: Early recognition of postoperative symptoms/signs of pSEH, warrant immediate MR examinations to diagnose the local/extent of hemorrhages. Subsequent emergent spinal decompressions/fusions are critical to limit/avert permanent postoperative neurological deficits. Additionally, patients undergoing open or minimally invasive spinal procedures where pSEH are suspected, warrant immediate postoperative MR studies.
Conclusion: Patients undergoing spinal surgery at any level typically become symptomatic from pSEH within 2.7 to 24 postoperative hours. Early recognition of new neurological deficits, immediate MR studies, and emergent surgery (i.e., if indicated) should limit/minimize postoperative neurological sequelae. Thus, pSEH should be treated, not ignored.
Keywords: Emergency, Diagnosis, Surgery, Postoperative spinal epidiural hematomas (pSEH), Not ignored, Avoid delays, Paralysis, Early diagnosis, Early surgery
INTRODUCTION
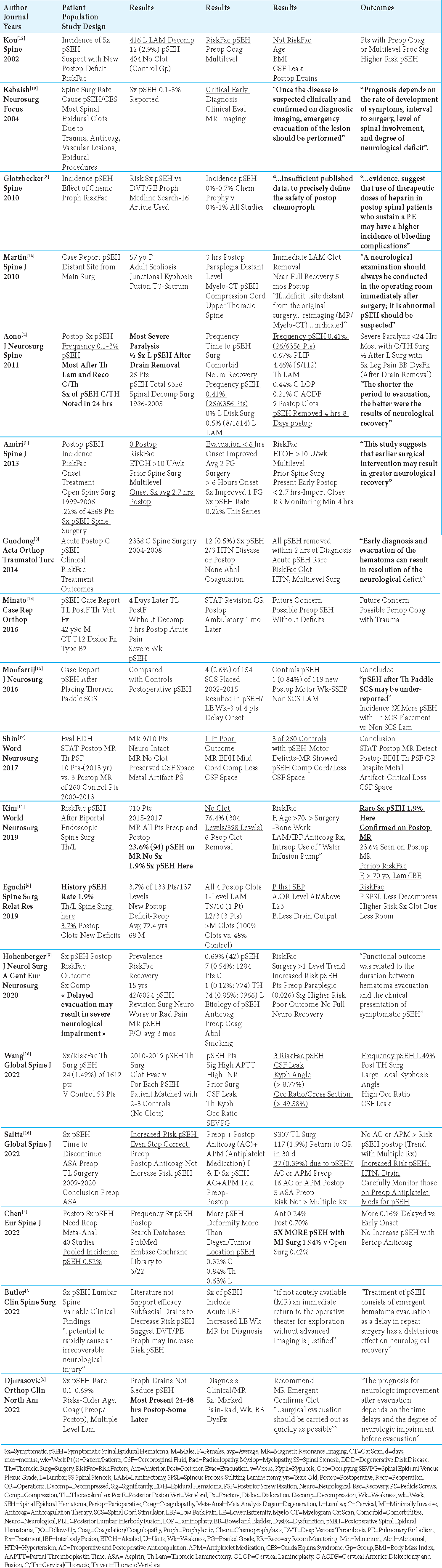
The incidence of symptomatic postoperative spinal epidural hematomas (pSEH) occurring at varying spinal levels ranged in 7 series from 0.1% to 1%,[
Incidence of pSEH
The incidence of symptomatic postoperative spinal epidural hematomas (pSEH) involving all spinal levels ranged in 7 studies from 0.1% to 1%,[
Multiple Risk Factors Associated with pSEH
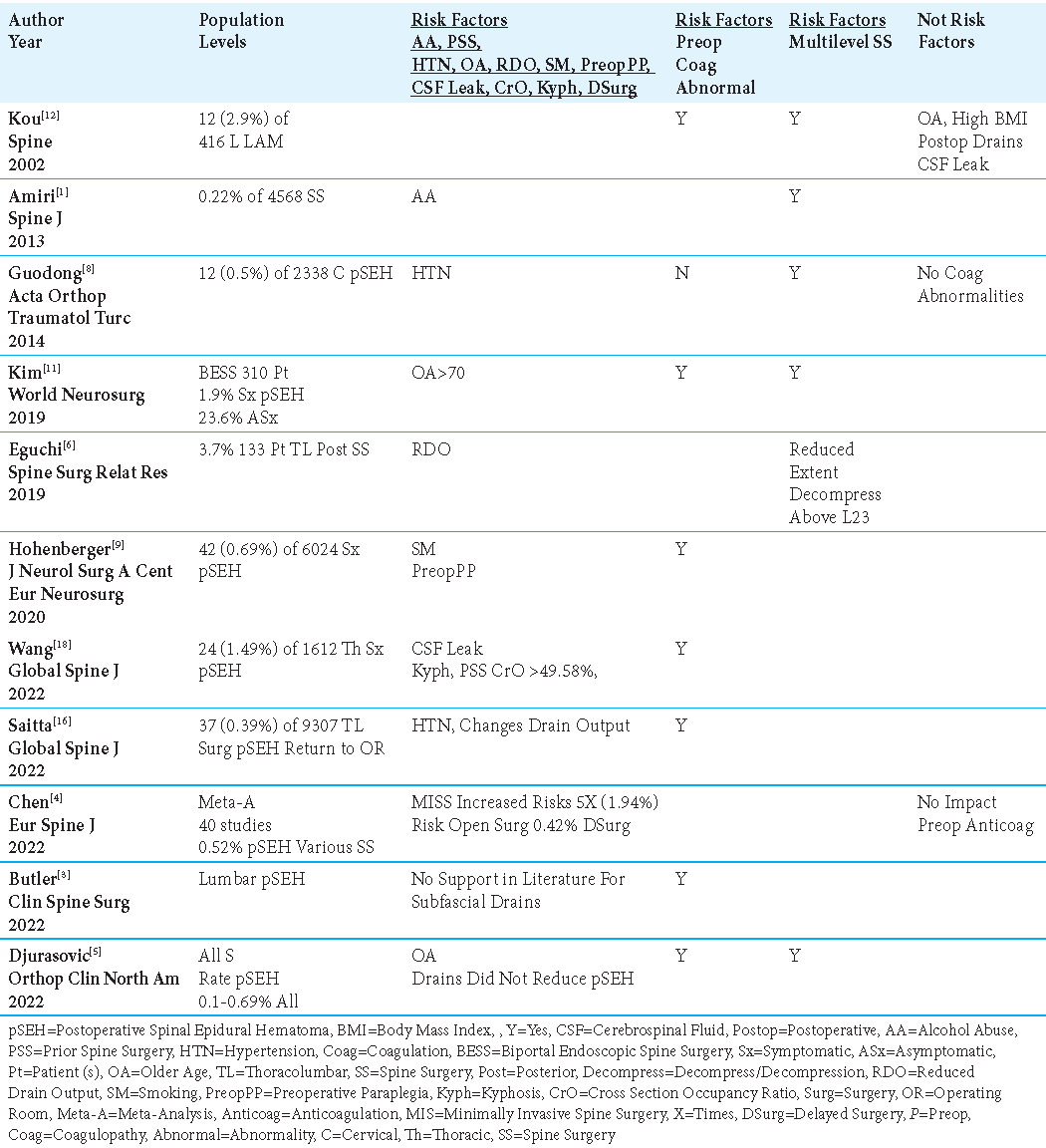
The most common risk factors predisposing patients to pSEH included; perioperative/postoperative coagulopathy,[
Controversy Regarding Correlation of Perioperative/Postoperative Coagulation Disorders with pSEH
Perioperative/Postoperative Coagulation Disorders Increased Risk of pSEH
In 6 spinal series, authors determined that preoperative/perioperative coagulation disorders/ coagulation abnormalities increased the risk of pSEH [
Perioperative Chemoprophylaxis for Deep Venous Thrombosis (DVT)/Pulmonary Embolism (PE) “May” Increase the Risk for pSEH
Two studies determined that chemoprophylaxis for Deep Venous Thrombosis (DVT)/Pulmonary Embolism (PE) “may” increase the risk for pSEH.[
Perioperative/Postoperative Coagulation Disorders/ Chemoprophylaxis Did Not Increase the Risk of pSEH
Two studies documented no increased risk of pSEH in patients with perioperative and/or postoperative coagulation abnormalities/disorders/chemoprophylaxis [
Minimally Invasive Surgery Increases Risk of pSEH 5-Fold
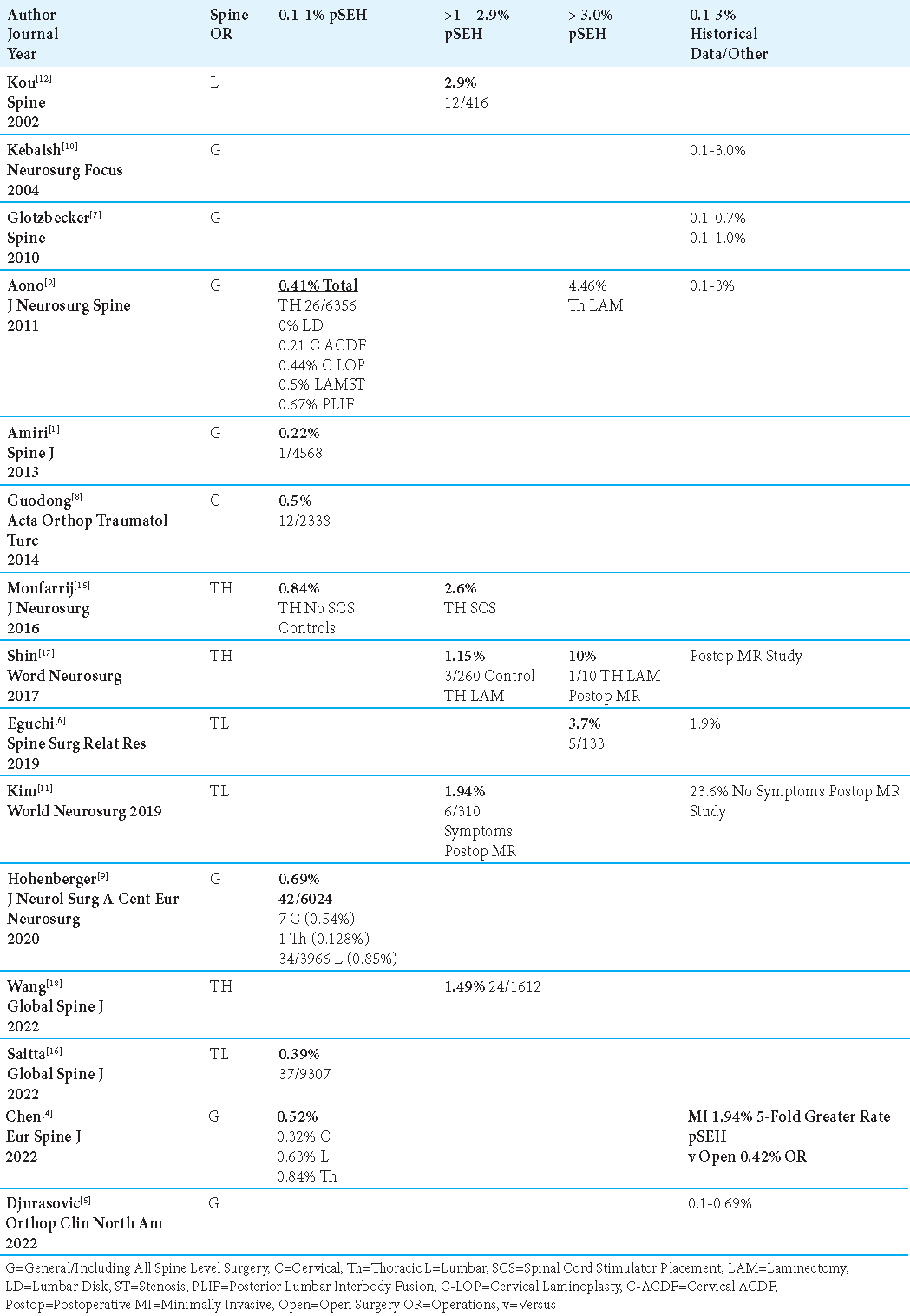
In Chen et al. (2022) meta-analysis of 40 studies, showing an overall 0.52% frequency of pSEH for all-level spinal procedures, they very specifically noted that minimally invasive (MI) operations increased the risk of pSEH 5-fold (1.94%) vs. open surgery (i.e. a lesser 0.425%) [
Placement of Thoracic Spinal Cord Stimulators (SCS) Increased Risk of pSEH 3-Fold
Moufarrij et al. (2016) found a 3-fold higher rate of pSEH for 4 (2.6%) of 154 patients undergoing SCS (i.e., placement of thoracic paddle leads for thoracic spinal cord stimulators through laminectomies); for the 119 control patients undergoing thoracic laminectomies without SCS placement, the frequency was a much lower 0.84% (i.e., 1 case of pSEH) [Tables 1and 4].[
Controversy Regarding Utility of Drains in Spinal Surgery to Avoid pSEH
Use of Drains to Monitor Output and Predict Onset of pSEH
Two studies advocated monitoring postoperative drainage following spine surgery to anticipate an increased risk for pSEH [
Drains In Spine Surgery Did Not Reduce the Incidence of pSEH
Three series concluded that placing drains during spine surgery did not reduce the frequency of pSEH [
Symptoms and Signs of pSEH
Several common symptoms and signs (i.e., acute increased postoperative pain, radiculopathy, weakness (i.e. including paraplegia), and sphincter dysfunction) signaled the presence/onset of pSEH following spinal surgery [
Acute pSEH can be Diagnosed on Immediate Postoperative MR Scans Despite Metal Artifact
MR scans are the “gold standard” for diagnosing immediate pSEH despite metallic artifact from instrumentation [
Gelfoam and/or Surgiflo If not Removed During Spinal Decompressions/Laminectomy May Swell/ Contribute to pSEH
Package inserts for both Gelfoam (i.e., Pharmacia and Upjohn Company 7000 Portage Road, Kalamazoo, Michigan 49001 USA), and Surgiflow Hemostatic Matrix (i.e. Ethicon, Johnson and Johnson 507 Mount Wellington Hwy, Penrose, New Jersey 1060, NZ) warn that these products should be removed if applied during spinal decompressions/laminectomies due to marked swelling (i.e., easily up to 20%). Although significant pSEH due to Gelfoam or Surgiflo can be readily diagnosed based on postoperative MR studies, spinal surgeons need to order these scans and perform definitive surgery in a timely fashion so that these products can be removed.
Gelfoam
The package insert for Gelfoam states; “...it (Gelfoam) should be removed after use in laminectomy procedures and from foramina in bone, once hemostasis is achieved. This is because GELFOAM may swell to its original size on absorbing fluids and produce nerve damage by pressure within confined bony spaces.” They further noted; “When GELFOAM was used in laminectomy operations, multiple neurologic events were reported, including but not limited to cauda equina syndrome, spinal stenosis, meningitis, arachnoiditis, headaches, paresthesias, pain, bladder and bowel dysfunction, and impotence”.
Surgiflo
The warnings for Surgiflo are simlar to those for Gelfoam; “SURGIFLO® should be removed from the site of application when used in, around, or in proximity to foramina in bone...”. “That is; “...because it may swell resulting in nerve damage”. Notably; “Safe and effective use in neurosurgery has not been proven...” Also; “SURGIFLO® may swell up to 20% upon contact with additional fluid...”, and; “...should be removed if possible once hemostasis has been achieved because of the possibility of dislodgment of the device or compression of other nearby anatomic structures.”
MR-Documentation of Spinal Levels of pSEH
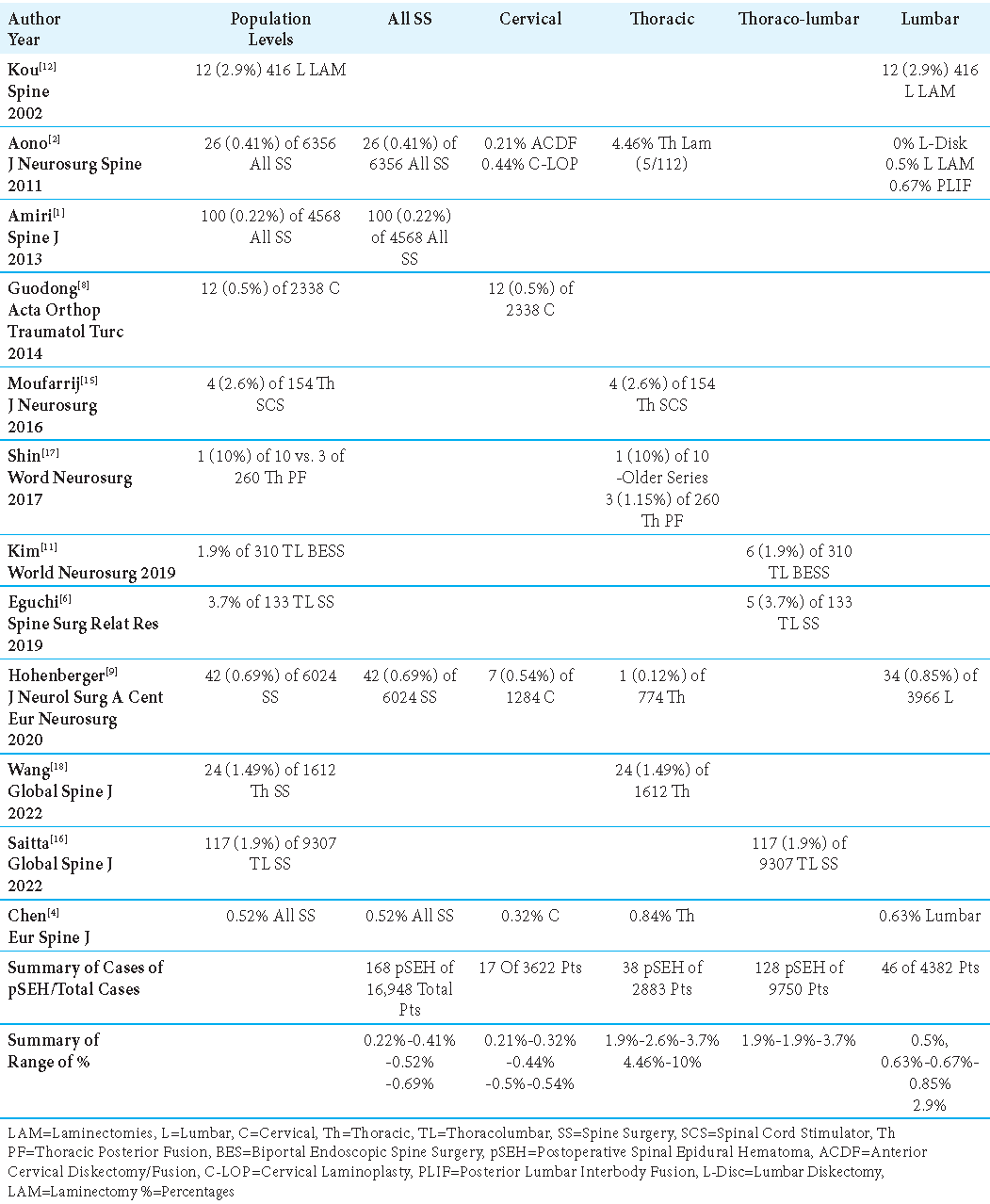
MR studies documented that most pSEH occurred in the thoracic spine, followed by the thoracolumbar spine, and cervical spine [
Timing to Onset of Symptoms from pSEH Following All-Level, Cervical, Thoracic, and Lumbar Surgery
Time to Onset of Postoperative Symptoms for pSEH After All-Level Spine Surgery
Four studies defined the times to onset of pSEH as ranging from 2.7 hrs., to < 24 hrs, to 24-48 hrs. after all-level spine surgery.[
Time to Onset of Postoperative Symptoms for Cervical pSEH
For patients undergoing cervical surgery, pSEH were typically symptomatic within < 24 postoperative hours.[
Time to Onset of Postoperative Symptoms for Thoracic/ Thoracolumbar pSEH
The following 3 series focused on the incidence of pSEH for patients undergoing thoracic/thoracolumbar surgery; patients became symptomatic from postoperative pSEH within 3 postoperative hrs. (i.e., 2 case reports), or within 24 hrs. postoperatively [
Time to Onset of Postoperative Symptoms for Lumbar pSEH
In Aono et al. (2011) series. 0.5% of patients undergoing lumbar laminectomies and 0.67% of those having PLIF became symptomatic from pSEH within 24 postoperative hrs. [
Early Diagnosis and Emergent Surgery Yielded the Best Outcomes for Patients with pSEH
This perspective emphasizes that early diagnosis and emergent surgery for patients with pSEH yielded the best neurological outcomes [
CONCLUSION
Symptomatic pSEH occur following 0.1%-4.46% of all spinal operations within an average of 2.7, to < 24 hrs (i.e., nearly 100% for cervical/thoracic, and 50% for lumbar lesions), to between 24-48 postoperative hrs. [
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Amiri AR, Fouyas IP, Cro S, Casey AT. Postoperative spinal epidural hematoma (SEH): Incidence, risk factors, onset, and management. Spine J. 2013. 13: 134-40
2. Aono H, Ohwada T, Hosono N, Tobimatsu H, Ariga K, Fuji T. Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. J Neurosurg Spine. 2011. 15: 202-5
3. Butler AJ, Donnally CJ, Goz V, Basques BA, Vaccaro AR, Schroeder GD. Symptomatic postoperative epidural hematoma in the lumbar spine. Clin Spine Surg. 2022. 35: 354-62
4. Chen Q, Zhong X, Liu W, Wong C, He Q, Chen Y. Incidence of postoperative symptomatic spinal epidural hematoma requiring surgical evacuation: A systematic review and meta-analysis. Eur Spine J. 2022. 31: 3274-85
5. Djurasovic M, Campion C, Dimar JR, Glassman SD, Gum JL. Postoperative epidural hematoma. Orthop Clin North Am. 2022. 53: 113-21
6. Eguchi Y, Suzuki M, Sato T, Yamanaka H, Tamai H, Kobayashi T. Post-operative spinal epidural hematoma after thoracic and lumbar spinous process-splitting laminectomy for thoracic and lumbar spinal stenosis. Spine Surg Relat Res. 2019. 3: 244-8
7. Glotzbecker MP, Bono CM, Wood KB, Harris MB. Postoperative spinal epidural hematoma: A systematic review. Spine (Phila Pa (1976). 2010. 35: E413-20
8. Guodong Y, Bin N. Acute postoperative cervical spinal epidural hematoma. Acta Orthop Traumatol Turc. 2014. 48: 437-42
9. Hohenberger C, Zeman F, Höhne J, Ullrich OW, Brawanski A, Schebesch KM. Symptomatic postoperative spinal epidural hematoma after spinal decompression surgery: Prevalence, risk factors, and functional outcome. J Neurol Surg A Cent Eur Neurosurg. 2020. 81: 290-6
10. Kebaish KM, Awad JN. Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus. 2004. 16: e1
11. Kim JE, Choi DJ, Kim MC, Park EJ. Risk factors of postoperative spinal epidural hematoma after biportal endoscopic spinal surgery. World Neurosurg. 2019. 129: e324-9
12. Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa (1976). 2002. 27: 1670-3
13. Martin CT, Kebaish KM. Postoperative spinal epidural hematoma at a site distant from the main surgical procedure: A case report and review of the literature. Spine J. 2010. 10: e21-5
14. Minato T, Miyagi M, Saito W, Shoji S, Nakazawa T, Inoue G. Spinal epidural hematoma after thoracolumbar posterior fusion surgery without decompression for thoracic vertebral fracture. Case Rep Orthop. 2016. 2016: 6295817
15. Moufarrij NA. Epidural hematomas after the implantation of thoracic paddle spinal cord stimulators. J Neurosurg. 2016. 125: 982-5
16. Saitta BH, Shultz P, Hanson K, Mikhael MM. Post-operative spinal epidural hematoma: are we discontinuing aspirin early enough?. Global Spine J. 2023. 13: 2255-61
17. Shin HK, Choi I, Roh SW, Rhim SC, Jeon SR. Relevance of postoperative magnetic resonance images in evaluating epidural hematoma after thoracic fixation surgery. World Neurosurg. 2017. 107: 803-8
18. Wang L, Wang H, Sun Z, Chen Z, Sun C, Li W. Incidence and risk factors for symptomatic spinal epidural hematoma following posterior thoracic spinal surgery in a single institute. Global Spine J. 2022. 12: 1175-83