- Professor of Clinical Neurosurgery, School of Medicine, State University of NY at Stony Brook and Editor-in-Chief Surgical Neurology International NY and c/o Dr. Marc Agulnick 1122 Franklin Avenue Suite 106, Garden City, New York, United States,
- Assistant Clinical Professor of Orthopedic Surgery, NYU Hospital Long Island c/o Dr. Marc Agulnick 1122 Franklin Avenue Suite 106, Garden City, NY, United States.
Correspondence Address:
Nancy E Epstein, Professor of Clinical Neurosurgery, School of Medicine, State University of NY at Stony Brook and Editor-in-Chief Surgical Neurology International NY and c/o Dr. Marc Agulnick 1122 Franklin Avenue Suite 106, Garden City, New York State, United States.
DOI:10.25259/SNI_710_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E Epstein1, Marc A Agulnick2. Perspective: Triple intraoperative neurophysiological monitoring (IONM) should be considered the standard of care (SOC) for performing cervical surgery for ossification of the posterior longitudinal ligament (OPLL). 15-Sep-2023;14:336
How to cite this URL: Nancy E Epstein1, Marc A Agulnick2. Perspective: Triple intraoperative neurophysiological monitoring (IONM) should be considered the standard of care (SOC) for performing cervical surgery for ossification of the posterior longitudinal ligament (OPLL). 15-Sep-2023;14:336. Available from: https://surgicalneurologyint.com/surgicalint-articles/12551/
Abstract
Background: Triple Intraoperative Neurophysiological Monitoring (IONM) should be considered the standard of care (SOC) for performing cervical surgery for Ossification of the Posterior Longitudinal Ligament (OPLL). IONM’s three modalities and their alerts include; Somatosensory Evoked Potentials (SEP: =/> 50% amplitude loss; =/>10% latency loss), Motor Evoked Potentials (MEP: =/> 70% amplitude loss; =/>10-15% latency loss), and Electromyography (loss of EMG, including active triggered EMG (t-EMG)).
Methods: During cervical OPLL operations, the 3 IONM alerts together better detect intraoperative surgical errors, enabling spine surgeons to immediately institute appropriate resuscitative measures and minimize/avoid permanent neurological deficits/injuries.
Results: This focused review of the literature regarding cervical OPLL surgery showed that SEP, MEP, and EMG monitoring used together better reduced the incidence of new nerve root (e.g., mostly C5 but including other root palsies), brachial plexus injuries (i.e., usually occurring during operative positioning), and/or spinal cord injuries (i.e., one study of OPLL patients documented a reduced 3.79% incidence of cord deficits utilizing triple IONM vs. a higher 14.06% frequency of neurological injuries occurring without IONM).
Conclusions: Triple IONM (i.e., SEP, MEP, and EMG) should be considered the standard of care (SOC) for performing cervical OPLL surgery. However, the positive impact of IONM on OPLL surgical outcomes critically relies on spinal surgeons’ immediate response to SEP, MEP, and/or EMG alerts/significant deterioration with appropriate resuscitative measures to limit/avert permanent neurological deficits.
Keywords: Anterior cervical surgery, Anterior corpectomy fusion (ACF), Anterior diskectomy/fusion (ACDF), Intraoperative neural monitoring (IONM), Surgical errors, Negligence, Somatosensory evoked potentials (SEP), Motor evoked potentials (MEP): Electromyography (EMG), Neurological injuries, Spinal cord deficits, C5 Palsy, Brachial plexus injury
INTRODUCTION
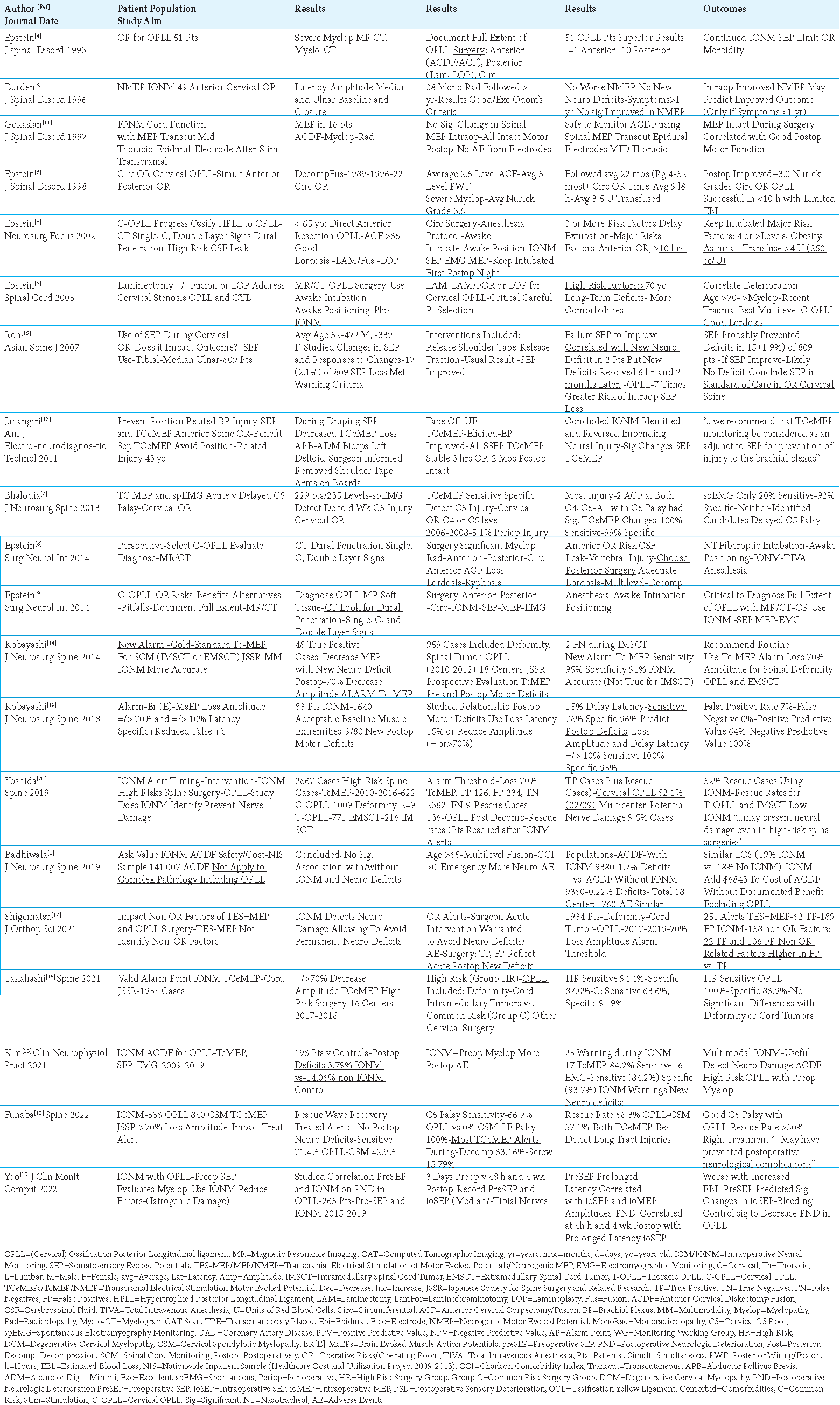
Triple Intraoperative Neurophysiological Monitoring (IONM) should be considered the standard of care (SOC) for performing cervical surgery for Ossification of the Posterior Longitudinal Ligament (OPLL). The three modalities of IONM and their alerts include; Somatosensory Evoked Potentials (SEP: =/> 50% amplitude loss; =/>10% latency loss), Motor Evoked Potentials (MEP: =/> 70% amplitude loss; =/>10-15% latency loss), and Electromyography (EMG: passive and active/triggered EMG (t-EMG)) [
Early Evidence that MEP were Safe/Effective for Monitoring ACDF for Disk Disease/Spondylosis/ Cervical Spondylotic Myelopathy
Two early studies showed that MEP were safe and provided effective IONM for anterior cervical surgery (i.e., ACDF for disk disease/spondylosis/cervical spondylotic myelopathy (CSM)) [
Evolution of IONM Techniques for Cervical OPLL Surgery From 1993-1998 Monitored with SEP/EMG to Combined SEP/EMG and MEP Monitoring by 2002-2014
For cervical OPLL surgery, Epstein documented the progression of IONM techniques from SEP/EMG monitoring alone from 1993-1998 to SEP/EMG and MEP techniques used together starting in 2002 [
Utility of SEP in Cervical OPLL Surgery
Roh et al. (2007) Used SEP as Part of the Standard of Care in Cervical Surgery, Especially for OPLL
In 2007, Roh et al. utilized SEP during 809 consecutive cervical operations [
Prolonged Preoperative SEP Latency Correlated with Intraoperative SEP/MEP Amplitude Losses and “Iatrogenic” Intraoperative and Postoperative Neurological Damage (PND)
Yoo et al. (2022) used SEP to reduce the incidence of intraoperative “iatrogenic damage” resulting in postoperative neurological damage (PND) observed in a series of 265 myelopathic patients undergoing cervical OPLL surgery [
SEP and TCeMEP Alerts Averted Brachial Plexus Injury in Anterior Cervical Surgery
While positioning for an anterior cervical procedure, Jahangir et al. (2011) found that SEP decreased, but TCeMEP were lost in the left upper extremity during draping for an anterior cervical procedure; the surgeon’s removal of the shoulder tape, and placement of the arms on arm boards resulted in SEP improvement, and full TCeMEP recovery [
TCeMEP Detect Cord Injuries/C5 Palsies in Cervical OPLL/CSM Surgical Patients, While EMG’s Were Less Accurate
Two studies documented TCeMEP detected cord/long tract motor deficits, and C5 palsies in patients undergoing cervical surgery for OPLL or CSM; EMG’s were less accurate [
Efficacy of IONM for ACDF in Patients with OPLL But Not Disc Disease/Spondylosis/CSM
IONM Not Effective in Anterior Diskectomy/Fusion (ACDF) Excluding OPLL Surgery
In 2019, Badhiwala et al. (2019) demonstrated that IONM did not limit/prevent neurological deficits when used in ACDF surgery performed for disc disease, spondylosis, or CSM patients; however, this series specifically excluded OPLL patients [
IONM Effective in ACDF Performed for Cervical OPLL
Kim et al. (2021) documented that IONM using SEP, TcMEP, and continuous EMG monitoring effectively averted/limited surgical errors/new neurological injuries from occurring during ACDF performed in patients with cervical OPLL [
MEP Alerts Warn of Surgical Errors/Impending Neurological Injury in Cervical OPLL and Other Cervical Surgery
Multiple studies effectively utilized IONM MEP alerts (i.e., set at =/> 70% decrease in amplitude; typically set at >/= 10% (i.e., subset set at >/= 15% decrease in latency)) to warn of surgical errors/impending neurological injuries occurring during OPLL, CSM and/or other cervical procedures [
CONCLUSION
This review indicates that triple IONM (i.e., SEP, MEP, and EMG) should be considered the standard of care (SOC) for performing cervical surgery for OPLL. Notably, using all 3 IONM modalities together better signaled intraoperative surgical errors that, if immediately addressed with appropriate resuscitative maneuvers, could limit/avert the onset of intraoperative/postoperative new neurological deficits (i.e. C5 and/or other root palsies, brachial plexus injuries, and/or new spinal cord deficits).
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Badhiwala JH, Nassiri F, Witiw CD, Mansouri A, Almenawer SA, da Costa L. Investigating the utility of intraoperative neurophysiological monitoring for anterior cervical discectomy and fusion: Analysis of over 140,000 cases from the National (Nationwide) Inpatient Sample data set. J Neurosurg Spine. 2019. 31: 76-86
2. Bhalodia VM, Schwartz DM, Sestokas AK, Bloomgarden G, Arkins T, Tomak P. Efficacy of intraoperative monitoring of transcranial electrical stimulation-induced motor evoked potentials and spontaneous electromyography activity to identify acute-versus delayed-onset C-5 nerve root palsy during cervical spine surgery: Clinical article. J Neurosurg Spine. 2013. 19: 395-402
3. Darden BV, Hatley MK, Owen JH. Neurogenic motor evoked-potential monitoring in anterior cervical surgery. J Spinal Disord. 1996. 9: 485-93
4. Epstein NE. The surgical management of ossification of the posterior longitudinal ligament in 51 patients. J Spinal Disord. 1993. 6: 432-54
5. Epstein NE. Circumferential surgery for the management of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1998. 11: 200-7
6. Epstein NE. Ossification of the cervical posterior longitudinal ligament: A review. Neurosurg Focus. 2002. 13: ECP1
7. Epstein NE. Laminectomy for cervical myelopathy. Spinal Cord. 2003. 41: 317-27
8. Epstein NE. Cervical surgery for ossification of the posterior longitudinal ligament: One spine surgeon’s perspective. Surg Neurol Int. 2014. 5: S88-92
9. Epstein NE. What you need to know about ossification of the posterior longitudinal ligament to optimize cervical spine surgery: A review. Surg Neurol Int. 2014. 5: S93-118
10. Funaba M, Kanchiku T, Yoshida G, Imagama S, Kawabata S, Fujiwara Y. Efficacy of intraoperative neuromonitoring using transcranial motor-evoked potentials for degenerative cervical myelopathy: A prospective multicenter study by the monitoring committee of the Japanese society for spine surgery and related research. Spine (Phila Pa 1976). 2022. 47: E27-37
11. Gokaslan ZL, Samudrala S, Deletis V, Wildrick DM, Cooper PR. Intraoperative monitoring of spinal cord function using motor evoked potentials via transcutaneous epidural electrode during anterior cervical spinal surgery. J Spinal Disord. 1997. 10: 299-303
12. Jahangiri FR, Holmberg A, Vega-Bermudez F, Arlet V. Preventing position-related brachial plexus injury with intraoperative somatosensory evoked potentials and transcranial electrical motor evoked potentials during anterior cervical spine surgery. Am J Electroneurodiagnostic Technol. 2011. 51: 198-205
13. Kim JE, Kim JS, Yang S, Choi J, Hyun SJ, Kim KJ. Neurophysiological monitoring during anterior cervical discectomy and fusion for ossification of the posterior longitudinal ligament. Clin Neurophysiol Pract. 2021. 6: 56-62
14. Kobayashi S, Matsuyama Y, Shinomiya K, Kawabata S, Ando M, Kanchiku T. A new alarm point of transcranial electrical stimulation motor evoked potentials for intraoperative spinal cord monitoring: A prospective multicenter study from the Spinal Cord Monitoring Working Group of the Japanese Society for Spine Surgery and Related Research. J Neurosurg Spine. 2014. 20: 102-7
15. Kobayashi K, Ando K, Shinjo R, Ito K, Tsushima M, Morozumi M. A new criterion for the alarm point using a combination of waveform amplitude and onset latency in Br(E)-MsEP monitoring in spine surgery. J Neurosurg Spine. 2018. 29: 435-41
16. Roh MS, Wilson-Holden TJ, Padberg AM, Park JB, Daniel Riew K. The utility of somatosensory evoked potential monitoring during cervical spine surgery: How often does it prompt intervention and affect outcome?. Asian Spine J. 2007. 1: 43-7
17. Shigematsu H, Yoshida G, Kobayashi K, Imagama S, Ando M, Kawabata S. Understanding the effect of non-surgical factors in a transcranial motor-evoked potential alert: A retrospective cohort study. J Orthop Sci. 2021. 26: 739-43
18. Takahashi M, Imagama S, Kobayashi K, Yamada K, Yoshida G, Yamamoto N. Validity of the alarm point in intraoperative neurophysiological monitoring of the spinal cord by the monitoring working group of the Japanese society for spine surgery and related research: A prospective multicenter cohort study of 1934 Cases. Spine (Phila Pa 1976). 2021. 46: E1069-76
19. Yoo M, Park YG, Cho YE, Lim CH, Chung SY, Kim D. Intraoperative evoked potentials in patients with ossification of posterior longitudinal ligament. J Clin Monit Comput. 2022. 36: 247-58
20. Yoshida G, Ando M, Imagama S, Kawabata S, Yamada K, Kanchiku T. Alert timing and corresponding intervention with intraoperative spinal cord monitoring for high-risk spinal surgery. Spine (Phila Pa 1976). 2019. 44: E470-9