- Department of Neurosurgery, Cork University Hospital, Wilton, Cork, Ireland
- Department of Neuropathology, Cork University Hospital, Wilton, Cork, Ireland
- Department of Otorhinolaryngology, South Infirmary Victoria University Hospital, Wilton, Cork, Ireland.
Correspondence Address:
Wail Salah Altaib, Department of Neurosurgery, Cork University Hospital, Wilton, Cork, Ireland.
DOI:10.25259/SNI_1107_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Wail Salah Altaib1, Mohamed Osman Dablouk1, Michael Jansen2, Mohammed Habibullah Khan3, Mahmoud Kamel1. Pituitary hyperplasia resulting in visual deficit. 24-Mar-2023;14:104
How to cite this URL: Wail Salah Altaib1, Mohamed Osman Dablouk1, Michael Jansen2, Mohammed Habibullah Khan3, Mahmoud Kamel1. Pituitary hyperplasia resulting in visual deficit. 24-Mar-2023;14:104. Available from: https://surgicalneurologyint.com/surgicalint-articles/12209/
Abstract
Background: Pituitary hyperplasia is an infrequent cause of visual disturbance and few such cases have been reported in the literature.
Case Description: We describe the case of a 16-year-old female who presented with a short history of progressive headache and visual blurring. Examination revealed markedly constricted visual fields. Imaging revealed an enlarged pituitary gland. Hormonal panel was normal. Following endoscopic endonasal transsphenoidal biopsy and decompression of the optic apparatus, an immediate improvement in vision was noted. Final histopathological examination revealed pituitary hyperplasia.
Conclusion: In patients with pituitary hyperplasia, visual deficit, and no identifiable reversible causes, surgical decompression can be considered to preserve vision.
Keywords: Hyperplasia, Pituitary, Transsphenoidal, Visual field
INTRODUCTION
Pituitary hyperplasia is an infrequent cause of visual disturbance and few such cases have been reported in the literature. While hyperplasia of the pituitary gland may occur due to pathology such as primary hypothyroidism or gonadal insufficiency,[
We describe the case of an adolescent female who presented with a short history of significant visual field loss due to pituitary hyperplasia. Following endoscopic endonasal transsphenoidal biopsy and decompression of the optic apparatus, an immediate improvement in vision was noted.
CASE PRESENTATION
History
A 16-year-old girl presented to the emergency department with a 10-day history of nonacute headache and associated binocular visual blurring. There was no associated nausea, vomiting, fatigue, or menstrual irregularity. Her medical history was significant for spina bifida, a lumbosacral dermoid tumor which had been operated twice in another institution, most recently 2 years before her current presentation, and hydrocephalus treated by ventriculoperitoneal shunt (now redundant), and subsequent endoscopic third ventriculostomy.
Examination
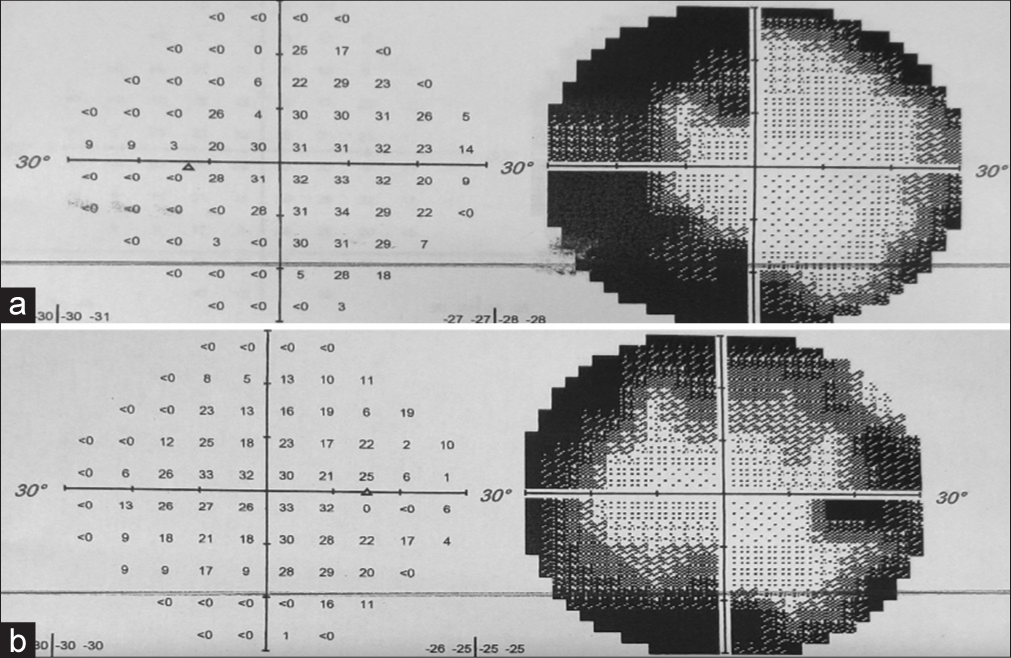
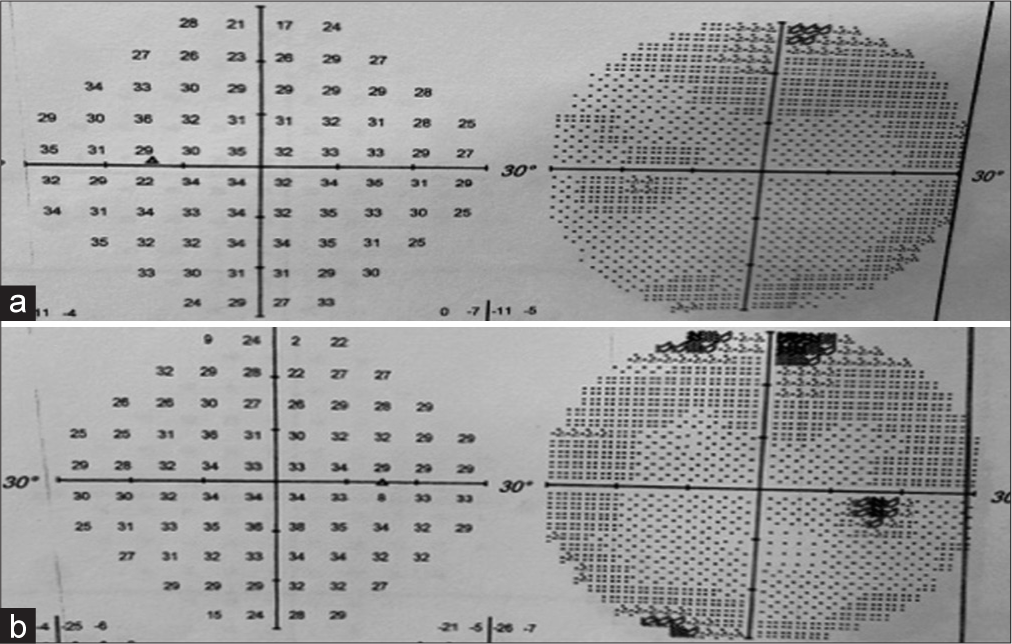
Neurological examination revealed a Glasgow coma score of 15/15 without any cranial nerve deficits, and no limb deficits apart from a pre-existing left lower limb weakness related to her previous spinal surgery. Formal ophthalmological examination revealed markedly constricted visual fields [
Investigations
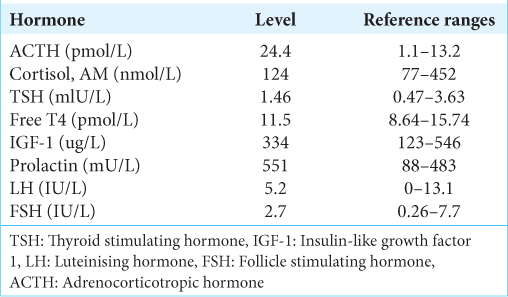
Serum investigations revealed a mildly elevated prolactin of 551 mU/L and an elevated adrenocorticotropic hormone (ACTH) level to 24.4 pmol/L with an otherwise normal hormonal panel [
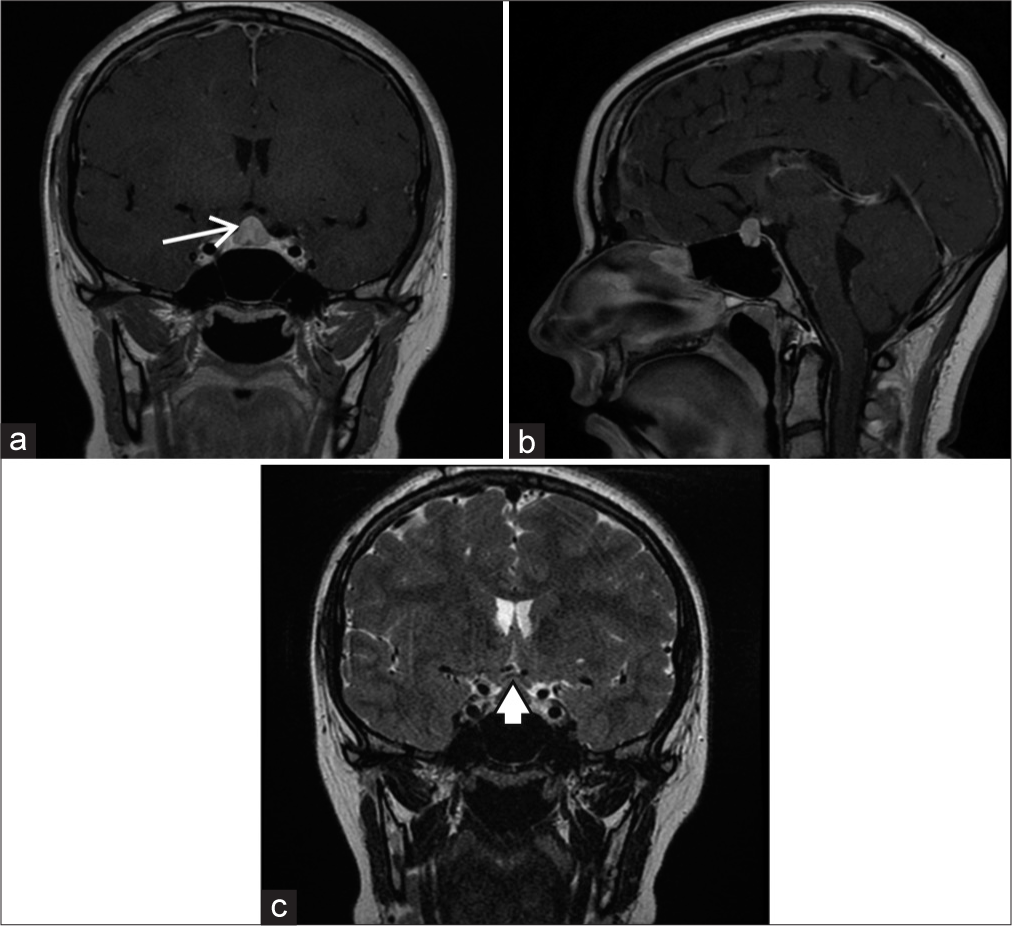
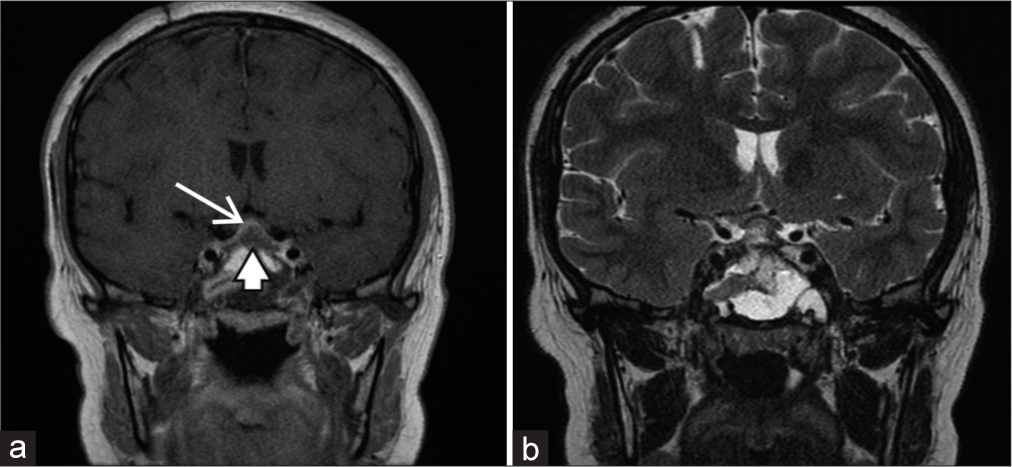
Magnetic resonance imaging (MRI) revealed an enlarged pituitary gland measuring 11.5 mm (CC) × 7 mm (AP) × 13 mm (transverse) [
Figure 2:
Preoperative magnetic resonance imaging. (a) Gadolinium-enhanced T1 coronal image showing enlarged pituitary gland displaying homogeneous enhancement (arrow); (b) gadolinium-enhanced T1 sagittal image; (c) T2-weighted coronal image showing upward displacement of the optic chiasm (arrowhead).
Operation
Following input from endocrinology and neurology, the patient was counseled regarding her options and debulking surgery was recommended with the aim of preserving vision. The patient and her guardian consented to surgery.
Following induction of general anesthesia, debulking of this lesion was undertaken through an endoscopic endonasal transphenoidal approach. Following septostomy and sphenoidotomy, the sellar floor was drilled and the dura opened. A firm lesion was noted and biopsies yielded an intraoperative pathological diagnosis of pituitary hyperplasia. The hyperplastic pituitary gland was dissected from the cavernous sinuses and suprasellar arachnoid mater to decompress the optic apparatus. Minimal cerebrospinal fluid leak was noted. The skull base was repaired using synthetic dural graft, autologous fat, and tissue glue.
Pathology
Initial evaluation of tissue through frozen section during surgery did not identify adenoma.
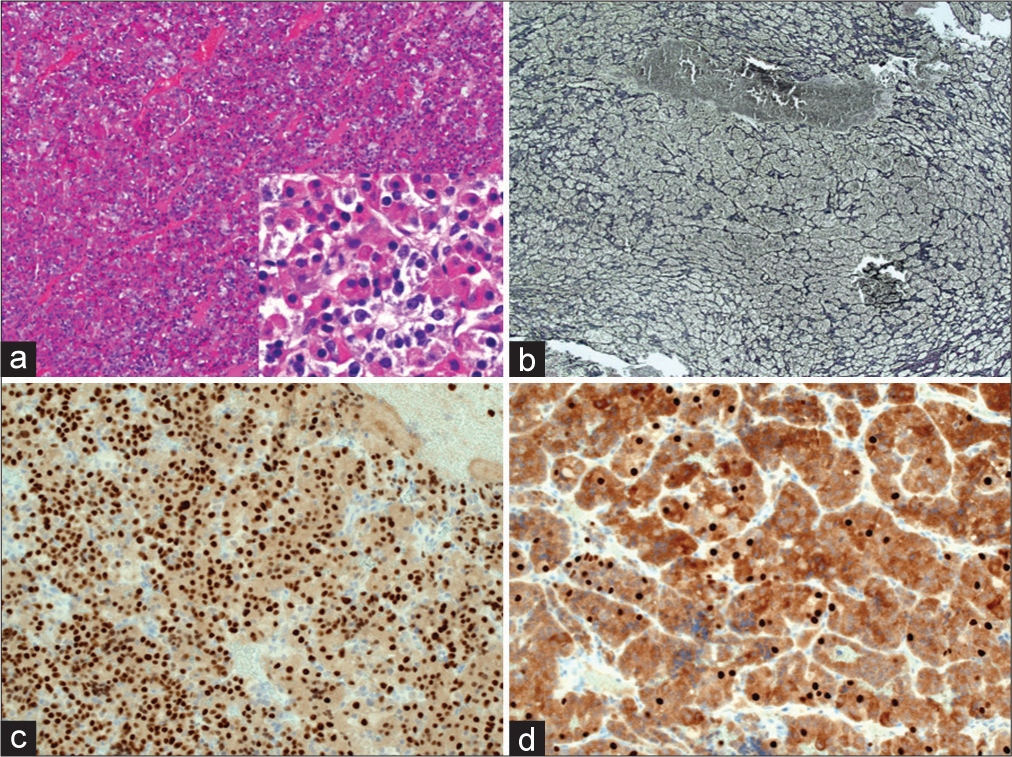
Further examination of the paraffin embedded tissue [
Figure 3:
(a) Pituitary hyperplasia (inset) various cell types present include eosinophilic, basophilic, and chromophoric cells. (b) Reticulin histochemistry – acinar structures outlined in black (Gomori’s modified reticulin stain) – expanded acini contrast with normally sized examples in the lower half of the photomicrograph. (c and d) Pit-1 immunostaining (Sigma polyclonal) - brown signal labels cell nuclei of Pit-lineage with more infrequent cell nuclei labelled with anti-SF-1 (ThermoFisher clone N1665).
Postoperative course
Postoperative MRI revealed adequate decompression of the optic chiasm with residual normal pituitary gland [
She required treatment with desmopressin for diabetes insipidus. Following endocrinology input, she was discharged home on maintenance desmopressin and hydrocortisone.
DISCUSSION
Within our unit, patients with pituitary adenomas who present with visual deficit and display evidence of mass effect on the optic apparatus are offered surgery, with the aim of surgical decompression and prevention of progressive visual deterioration. A clear management paradigm for patients with pituitary hyperplasia and visual deficit does not exist and there are few reports of such cases.
Raviv et al.[
Kinoshita et al.[
On MRI, a pituitary adenoma can often be differentiated from the normal pituitary gland on close examination. Pituitary hyperplasia, however, does not appear as a discrete lesion and instead is associated with an increased overall volume of the pituitary gland. Pituitary size may vary due to size and age. In our patient, the pituitary gland volume was calculated to be 544 mm3,[
Notably, our patient’s laboratory investigations revealed an elevated ACTH. Although the precise mechanism for this is uncertain, we hypothesize that her previous history of multiple courses of dexamethasone therapy as part of treatment of her lumbosacral dermoid tumor may have played a role; hypoadrenocorticism from steroid therapy is well described and has been known to cause elevated ACTH levels but normal levels of serum cortisol.[
To the best of our knowledge, this is the second reported case of profound visual disturbance related to physiological pituitary hyperplasia in which the visual deficit resolved with surgical decompression.
The previous authors have hypothesized that pituitary hyperplasia may be more likely to cause visual deficit than pituitary adenomas due to the relatively firm nature of the tissue,[
Given that little is known about the natural history of such presentations and the few reported cases in the literature, it is difficult to know how to optimally manage such patients. In both our case and in the case described by Raviv et al.,[
With this case, we have added to the currently small body of literature which suggests that in patients with pituitary hyperplasia, visual deficit, and no identifiable reversible causes, surgical decompression can be considered to preserve and/or improve vision. There is not yet sufficient experience to suggest the optimal surgical strategy; however, opening of the sellar bone and dura of the sellar floor with dissection of the pituitary from the surrounding dura and arachnoid layers have been seen to provide benefit in the cases described to date.
Written consent was obtained from the patient before the writing of this case report. All identifying information has been removed. Ethical approval was not deemed applicable in this case.
CONCLUSION
In patients with pituitary hyperplasia, visual deficit and no identifiable reversible causes, surgical decompression can be considered to preserve vision.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. De Sousa SM, Earls P, McCormack AI. Pituitary hyperplasia: Case series and literature review of an under-recognised and heterogenous condition. Endocrinol Diabetes Metab Case Rep. 2015. 2015: 150017
2. Jameson JL, De Groot LJ, editors. Endocrinology: Adult and Pediatric E-Book. Netherlands: Elsevier Health Sciences; 2015. p.
3. Kinoshita Y, Fumiyuki Y, Tominaga A, Usui S, Kurisu K. Physiologic pituitary hyperplasia causing visual disturbance during adolescence. J Clin Neurosci. 2019. 61: 279-81
4. Raviv N, Amin A, Kenning TJ, Pinheiro-Neto CD, Jones D, Sharma V. Pituitary hyperplasia causing complete bitemporal hemianopia with resolution following surgical decompression: Case report. J Neurosurg. 2020. p. 1-5
5. Yadav P, Singhal S, Chauhan S, Harit S. MRI evaluation of size and shape of normal pituitary gland: Age and sex related changes. J Clin Diagn Res. 2017. 11: TC01-4
6. Zhou J, Ruan L, Li H, Wang Q, Zheng F, Wu F. Addison’s disease with pituitary hyperplasia: A case report and review of the literature. Endocrine. 2009. 35: 285-9