- Department of Neurosurgery, Toyama University, Toyama, Japan
Correspondence Address:
Yuichiro Koga, Department of Neurosurgery, Toyama University, Toyama, Japan
DOI:10.25259/SNI_233_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yuichiro Koga, Takuya Akai, Taisuke Shiro, Satoshi Kuroda. Pituitary lymphoma appearing 9 years after pituitary adenoma resection. 26-Jul-2024;15:262
How to cite this URL: Yuichiro Koga, Takuya Akai, Taisuke Shiro, Satoshi Kuroda. Pituitary lymphoma appearing 9 years after pituitary adenoma resection. 26-Jul-2024;15:262. Available from: https://surgicalneurologyint.com/surgicalint-articles/13008/
Abstract
Background: Pituitary lymphomas (PLs) are very rare, accounting for
Case Description: A 51-year-old woman presented with visual disturbance. She had a history of pituitary adenoma resected through endoscopic trans-sphenoidal surgery (eTSS) 9 years before. Although her previous annual follow-up did not show any signs of recurrence, she noticed visual disturbance. One month later, her visual acuity rapidly worsened with headache and fatigue, being referred to our hospital. On examination, she had bilateral quadrantanopia. Her laboratory data showed slightly increased prolactin levels. Magnetic resonance images showed a mass in the sella with suprasellar extension, so she underwent eTSS. The tumor had a fibrous, hard part and a soft gray part, and it was mostly resected. Visual symptoms improved transiently, but ophthalmoplegia appeared 2 weeks after surgery, indicating intrathecal dissemination. Histological analysis confirmed the diagnosis of T-lymphoblastic lymphoma. Positron emission tomography showed tracer accumulation at the pancreas, confirmed as lymphoma through biopsy. However, we could not determine which site of lymphoma was the primary site. She underwent chemotherapy, including cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, dexamethasone, and methotrexate. The patient died despite several months of treatment.
Conclusion: Recurrence of pituitary adenoma cannot be carelessly assumed from a pituitary growing mass after pituitary adenoma resection. PLs have poor prognosis due to their aggressive character. Immediate biopsy and confirmation of the diagnosis are necessary for the treatment of pituitary masses with aggressive features.
Keywords: Endoscopic trans-sphenoidal surgery, Pituitary adenoma, Pituitary lymphoma, Recurrence, T-lymphoblastic lymphoma
INTRODUCTION
Pituitary tumors account for 15% of all intracranial tumors.[
CLINICAL SUMMARY
A 51-year-old woman presented to our hospital with blurred vision and a visual field defect. Nine years before the presentation, she visited the hospital due to visual disturbance. Her magnetic resonance (MR) images showed a mass in the sellar region compressing the chiasma [
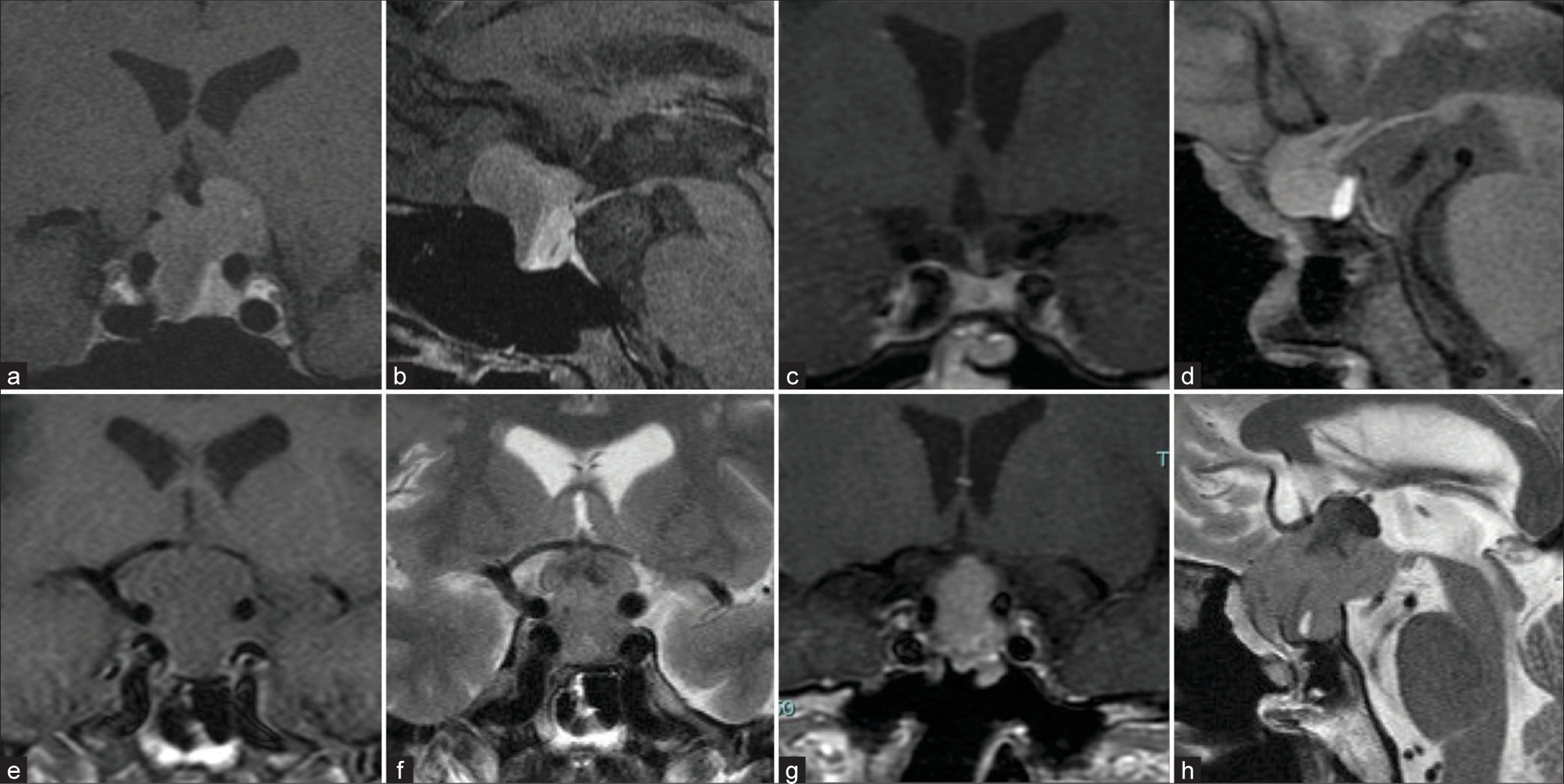
Figure 1:
(a-d) Gadolinium-enhanced magnetic resonance (MR) images before the 1st operation and 1 year before the presentation. The heterogeneously enhanced tumor is observed in the sella with suprasellar extension before surgery. No tumor recurrence is apparent at 8 years after surgery. MR images at presentation. An extensively enlarged tumor in the sellar and suprasellar portion with optic nerve compression. (e-h) The tumor is shown isointense on T1-weighted images (WI), having iso-low mixed intensity on T2-WI, and heterogeneously enhanced by gadolinium.
A year before the presentation, she did not have any symptoms, and her MR images did not show tumor recurrence [
Figure 2:
(a: Hematoxylin and eosin (H&E) stain, Magnifications×400) Histological analysis of resected tumor tissue. Tumor cells have round hyperchromatic nuclei and sparse eosinophilic cytoplasm. No apparent necrotic lesion is observed. (b: CD3, c: CD4, d: CD8, e: CD20, f: CD56, g: TdT) Immunohistochemical analysis reveals that tumor cells were positive for CD3, CD4, CD8, and terminal deoxynucleotidyl transferase (TdT) and negative for CD20 and CD56 Magnifications: ×400. Scale bars: 20 μm (a-g).
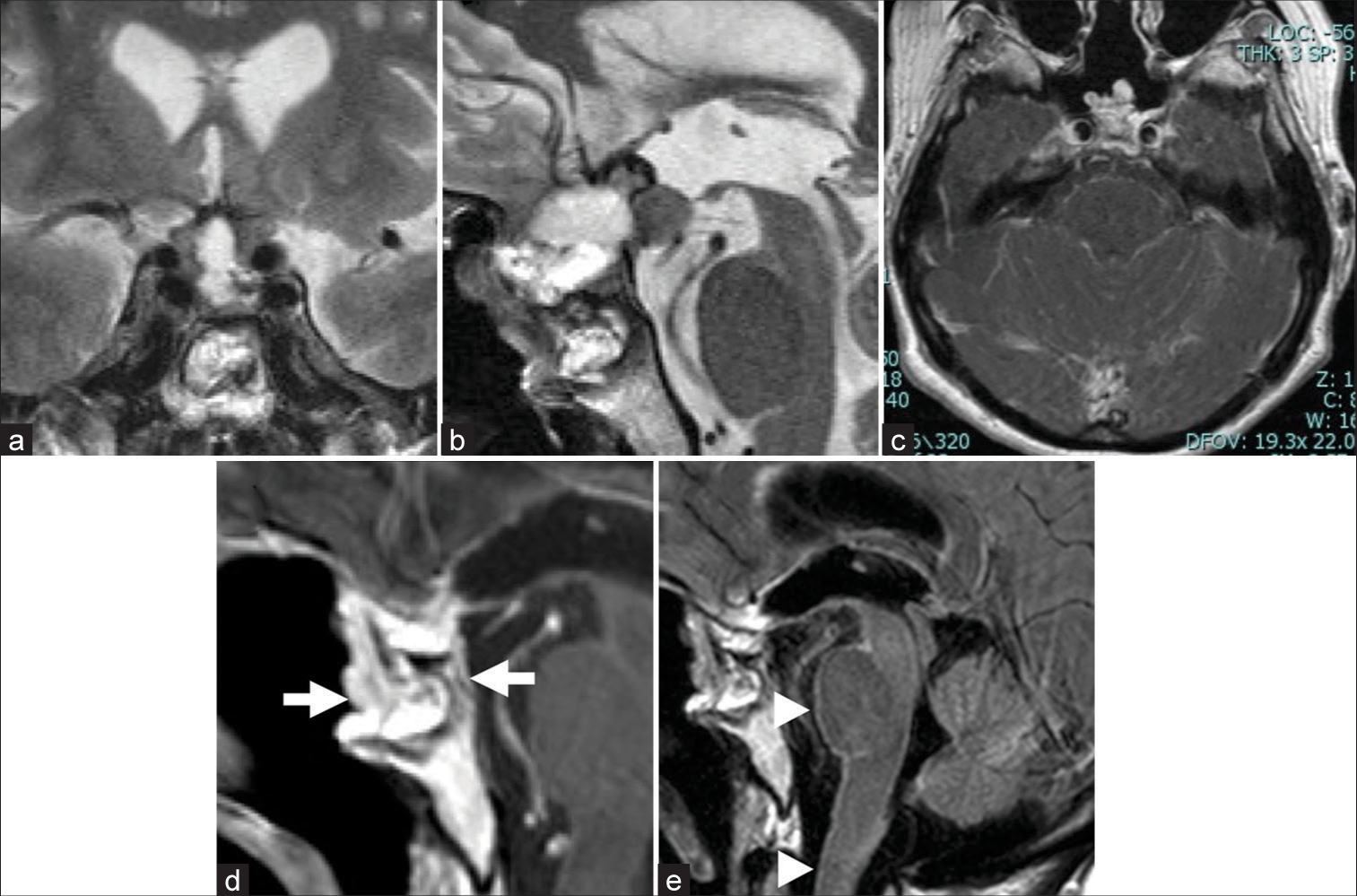
Two weeks after surgery, she experienced ptosis on her right eyelid, ophthalmoplegia, dysarthria, and dysphagia. MR images showed pial enhancement in the brain stem in a fluid-attenuated inversion recovery image, diagnosed as tumor meningeal dissemination [
Figure 3:
(a and b) Postoperative magnetic resonance (MR) images. T2-weighted image (WI) shows the residual tumor attached to the right cavernous sinus and suprasellar portion ([a] Coronal, [b] Sagittal). (c) Gadolinium-enhanced MR fluid-attenuated inversion recovery image taken 14 days after the second surgery shows pial enhancement on the brain stem and cerebellum and indicates intrathecal dissemination. (d and e) Gadolinium-enhanced T1-WI after chemoradiotherapy (11 months after the second surgery) shows an enhanced region in the sellar portion and clivus (arrows) and pial enhancement from the brain stem to the spinal cord (arrowheads).
DISCUSSION
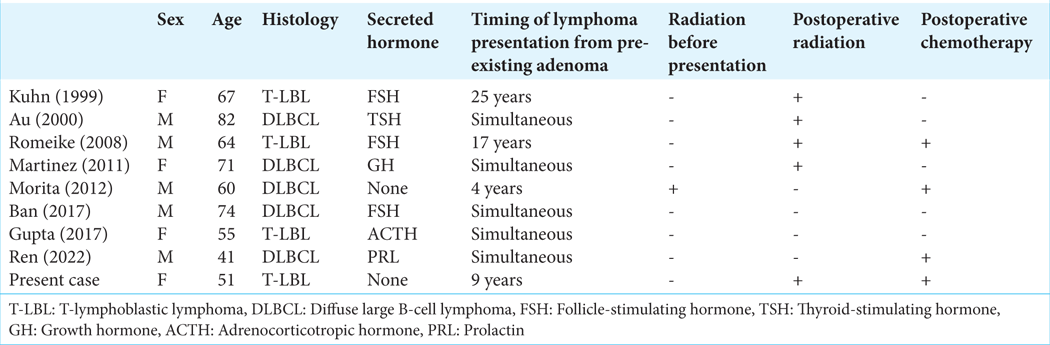
PL, whether primary or metastatic, is very rare, accounting for <1% of all intracranial tumors. PL can arise de novo or in association with pituitary adenomas or lymphocytic hypophysitis.[
The neurological manifestation of PL resembles pituitary adenoma due to its dependency on tumor location and its extension. For example, common physical manifestations include visual acuity decline, temporal hemianopsia, diplopia, and headache. Pituitary insufficiency can also be commonly seen. Cranial nerve (CN) palsies retroorbital pain may be observed in case of extension into the cavernous sinus or orbital apex. Due to anatomical localization, CN II is most involved, followed by CN III, VI, and, less frequently, CN V, IV, and VII. Endocrinological manifestations such as hypopituitarism and diabetes insipidus (DI) appear to be more common in PLs than in pituitary adenomas.[
The pathophysiology of lymphoma arising in the Sella is still controversial; it is unclear whether PL is derived from adjacent meningeal lymphoid tissue or is caused by the malignant transformation of normal lymphocytes migrating into the sella during inflammation.[
Treatment of PLs often includes surgical resection, chemotherapy, and radiotherapy. In most cases, surgical resection through eTSS is performed. Total or subtotal tumor resection increases more overall survival and progression-free survival than biopsy alone.[
The present patient had a single tumor in the pancreas, confirmed by biopsy to be the same type of lymphoma in the sella. We could not fully determine whether the PL in our case was a primary lesion or due to metastasis. However, metastasis in the pituitary gland occurs mostly in the posterior lobe with manifesting DI.[
CONCLUSION
Herein, we reported a case of PLPA. PLPA has a poor prognosis due to its aggressive character. Recurrence cannot be assumed from a pituitary growing mass after pituitary adenoma resection. Immediate confirmation of the diagnosis by biopsy and general examination is necessary in case of a pituitary mass with aggressive features. Further studies are necessary to clarify the pathogenesis of lymphoma cell’s invasion into the pituitary gland.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdelbaset-Ismail A, Borkowska S, Janowska-Wieczorek A, Tonn T, Rodriguez C, Moniuszko M. Novel evidence that pituitary gonadotropins directly stimulate human leukemic cells-studies of myeloid cell lines and primary patient AML and CML cells. Oncotarget. 2016. 7: 3033-46
2. Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO classification of pituitary tumors. Endocr Pathol. 2022. 33: 6-26
3. Au WY, Kwong YL, Shek TW, Leung G, Ooi C. Diffuse large-cell B-cell lymphoma in a pituitary adenoma: An unusual cause of pituitary apoplexy. Am J Hematol. 2000. 63: 231-2
4. Ban VS, Chaudhary BR, Allinson K, Santarius T, Kirollos RW. Concomitant primary CNS lymphoma and FSH-pituitary adenoma arising within the sella. Entirely coincidental? Neurosurgery. 2017. 80: E170-5
5. Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M. Primary intracerebral malignant lymphoma: Report of 248 cases. J Neurosurg. 2000. 92: 261-6
6. Braesch-Andersen S, Paulie S, Stamenkovic I. Dopamine-induced lymphoma cell death by inhibition of hormone release. Scand J Immunol. 1992. 36: 547-53
7. Costa O, Bouthet C, Sauvage P, Michel JP, Deschaux P. Age-dependent LH and FSH effect on the proliferation of women’s peripheral blood lymphocytes in vitro. Int J Immunopharmacol. 1990. 12: 821-9
8. Deak D, Gorcea-Andronic N, Sas V, Teodorescu P, Constantinescu C, Iluta S. A narrative review of central nervous system involvement in acute leukemias. Ann Transl Med. 2021. 9: 68
9. Famini P, Maya MM, Melmed S. Pituitary magnetic resonance imaging for sellar and parasellar masses: Ten-year experience in 2598 patients. J Clin Endocrinol Metab. 2011. 96: 1633-41
10. Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am. 1999. 28: 81-117.vi
11. Giustina A, Gola M, Doga M, Rosei EA. Clinical review 136: Primary lymphoma of the pituitary: An emerging clinical entity. J Clin Endocrinol Metab. 2001. 86: 4567-75
12. Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998. 19: 717-97
13. Gupta RK, Saran RK, Srivastava AK, Jagetia A, Garg L, Sharma MC. T cell lymphoblastic lymphoma/leukemia within an adrenocorticotropic hormone and thyroid stimulating hormone positive pituitary adenoma: A cytohistological correlation emphasizing importance of intra-operative squash smear. Neuropathology. 2017. 37: 358-64
14. Ilahi S, Ilahi TB, editors. Anatomy, adenohypophysis (pars anterior, anterior pituitary). StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. p.
15. Jain D, Sharma MC, Sarkar C, Suri V, Garg A, Mahapatra AK. Pituitary gland involvement by a gamma delta hepatosplenic lymphoma, a mimicker of pituitary adenoma: Report of a rare case. J Neurooncol. 2008. 88: 237-41
16. Kern WF, Spier CM, Hanneman EH, Miller TP, Matzner M, Grogan TM. Neural cell adhesion molecule-positive peripheral T-cell lymphoma: A rare variant with a propensity for unusual sites of involvement. Blood. 1992. 79: 2432-7
17. Kuhn D, Buchfelder M, Brabletz T, Paulus W. Intrasellar malignant lymphoma developing within pituitary adenoma. Acta Neuropathol. 1999. 97: 311-6
18. Martinez JH, Martinez MD, de Gorgola MM, Montalvo LF, Tome JE. The coexistence of an intrasellar adenoma, lymphocytic hypophysitis, and primary pituitary lymphoma in a patient with acromegaly. Case Rep Endocrinol. 2011. 2011: 941738
19. März M, Meyer S, Erb U, Georgikou C, Horstmann MA, Hetjens S. Pediatric acute lymphoblastic leukemia-Conquering the CNS across the choroid plexus. Leuk Res. 2018. 71: 47-54
20. Matera L, Cutufia M, Geuna M, Contarini M, Buttiglieri S, Galin S. Prolactin is an autocrine growth factor for the Jurkat human T-leukemic cell line. J Neuroimmunol. 1997. 79: 12-21
21. Mendes-da-Cruz DA, Belorio EP, Cotta-de-Almeida V. Thymus-brain connections in T-cell acute lymphoblastic leukemia. Neuroimmunomodulation. 2024. 31: 51-61
22. Morita K, Nakamura F, Kamikubo Y, Mizuno N, Miyauchi M, Yamamoto G. Pituitary lymphoma developing within pituitary adenoma. Int J Hematol. 2012. 95: 721-4
23. Papanastasiou L, Pappa T, Dasou A, Kyrodimou E, Kontogeorgos G, Samara C. Case report: Primary pituitary non-Hodgkin’s lymphoma developed following surgery and radiation of a pituitary macroadenoma. Hormones (Athens). 2012. 11: 488-94
24. Ren S, Lu Q, Xiao Y, Zhang Y, Zhang L, Li B. Coexistence of pituitary adenoma and primary pituitary lymphoma: A case report and review of the literature. Front Surg. 2022. 9: 842830
25. Romeike BF, Joellenbeck B, Stein H, Loddenkemper C, Hummel M, Firsching R. Precursor T-lymphoblastic lymphoma within a recurrent pituitary adenoma. Acta Neurochir (Wien). 2008. 150: 833-6
26. Scharff B, Modvig S, Marquart HV, Christensen C. Integrin-mediated adhesion and chemoresistance of acute lymphoblastic leukemia cells residing in the bone marrow or the central nervous system. Front Oncol. 2020. 10: 775
27. Tamer G, Kartal I, Aral F. Pituitary infiltration by nonHodgkin’s lymphoma: A case report. J Med Case Rep. 2009. 3: 9293
28. Tarabay A, Cossu G, Berhouma M, Levivier M, Daniel RT, Messerer M. Primary pituitary lymphoma: An update of the literature. J Neurooncol. 2016. 130: 383-95
29. Thastrup M, Duguid A, Mirian C, Schmiegelow K, Halsey C. Central nervous system involvement in childhood acute lymphoblastic leukemia: Challenges and solutions. Leukemia. 2022. 36: 2751-68
30. Weller M, Martus P, Roth P, Thiel E, Korfel A. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol. 2012. 14: 1481-4