- Department of Medicine, Faculdade Israelita de Ciências da Saúde Albert Einstein, São Paulo, Brazil,
- Neurosurgical Service, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA,
- Department of Neurosurgery, Mayo Clinic, Jacksonville, Florida, United States,
- Department of Interventional Neuroradiology, Hospital Beneficência Portuguesa de São Paulo, São Paulo, Brazil.
Correspondence Address:
Rafael Trindade Tatit, Department of Medicine, Faculdade Israelita de Ciências da Saúde Albert Einstein, São Paulo, Brazil.
DOI:10.25259/SNI_1139_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rafael Trindade Tatit1, Christopher S. Ogilvy2, Max S. Shutran2, Rabih G. Tawk3, Thomas A. Yasuda4, Carlos Eduardo Baccin4. Plasticity of the adult circle of Willis in response to flow diversion stents. 10-Feb-2023;14:49
How to cite this URL: Rafael Trindade Tatit1, Christopher S. Ogilvy2, Max S. Shutran2, Rabih G. Tawk3, Thomas A. Yasuda4, Carlos Eduardo Baccin4. Plasticity of the adult circle of Willis in response to flow diversion stents. 10-Feb-2023;14:49. Available from: https://surgicalneurologyint.com/surgicalint-articles/12149/
Abstract
Background: We present five patients with remodeling of the adult circle of Willis in response to flow diverter stents (FDSs) at the anterior communicating artery (AComA) and the posterior communicating artery (PComA). The observed changes provide a paradigm of how flow change can institute anatomic changes in the adult circle of Willis vasculature.
Case Description: In the first two cases, after placement of the FDS covering the AComA, there was an increase in size and flow of the contralateral A1-anterior cerebral artery which had previously been hypoplastic. In one of the cases, this led to the filling of the aneurysm and required placement of coils within the lesion which was curative. In case three, the FDS effect led to asymptomatic occlusion of the PComA and associated aneurysm without change of the ipsilateral P1-segement of posterior-cerebral-artery (P1-PCA) caliber. In the fourth case, the FDS covering an aneurysm with a fetal PCA arising from its neck resulted in significant reduction of the aneurysm size, persistent flow and caliber of the fetal PCA, and the hypoplastic ipsilateral P1-PCA. Finally, in the fifth case, after FDS occlusion of the PComA and aneurysm there was increasement in diameter of the ipsilateral P1-PCA that was previously hypoplastic.
Conclusion: The use of FDS can affect vessels covered by the device and other arteries of the circle of Willis adjacent to the FDS. The phenomena illustrated in the hypoplastic branches appear to be a compensatory response to the hemodynamic changes induced by the divertor and to the altered flow in the circle of Willis.
Keywords: Circle of Willis, Flow diversion, Intracranial aneurysms, Vascular remodeling
INTRODUCTION
Flow diverters have been established as safe and first-line devices for carefully selected intracranial aneurysms (IAs), representing more than a third of aneurysms treated in some centers.[
CASES REPORTS
Patient 1
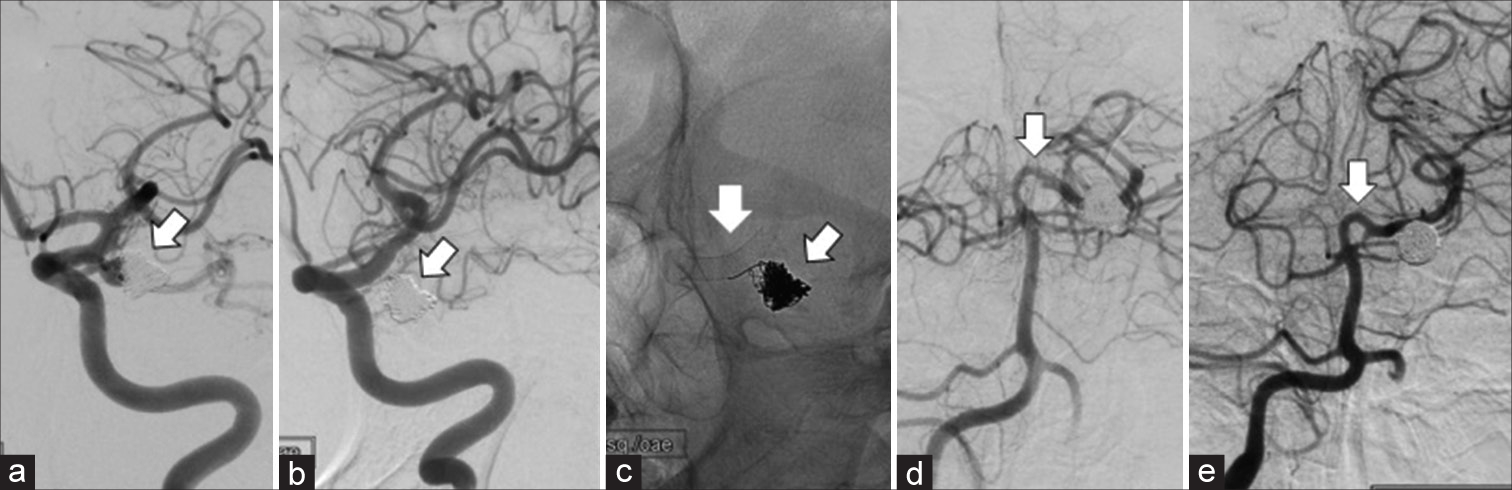
A 54-year-old man with anterior communicating artery (AComA) IA was initially treated with a pipeline flow-diverter implanted in the left anterior cerebral artery (ACA), jailing the AComA. In the control of 12 months, it was noted that the contralateral A1 segment of the ACA (A1-ACA) was hypoplastic initially. This vessel increased in diameter and recanalized the previously treated aneurysm. This led to the decision to embolize AComA IA with coils, accessing it through the contralateral ACA, which resulted in complete obliteration of the lesion which has remained stable in follow-up studies over 1 year [
Figure 1:
(a) Initial cerebral angiography image demonstrating hypoplastic A1-anterior cerebral artery (ACA) with minimal filling of right ACA (arrow). (b and c) Cerebral angiography image performed 26 months after coils and pipeline. Note increased caliber of the right A1 and filling of the ACA with a stable aneurysm occlusion (arrows). (d) Plain X-ray showing the flow diverter stents and coils (arrow).
Patient 2
A 71-year-old woman with AComA IA was treated with a pipeline flow-diverter implanted in the left ACA from the A1 segment to the A2 segment, excluding the AComA. In the follow-up control angiogram at 2 years after, it was noted that the A1-ACA on the right had increased in diameter. However, in this case, there was no recanalization of the IA, which was completely obliterated [
Figure 2:
(a) Initial cerebral angiography image demonstrating a hypoplastic right A1-anterior cerebral artery (ACA) (arrow) without filling of the ipsilateral ACA. (b and c) Cerebral angiography follow-up almost 24 months after placement of the flow diverter stents in the left A1-A2 shows increased caliber of the right A1 segment and now filling of the right ACA (arrow). The aneurysm remained occluded (arrow). (d) Initial cerebral angiography image demonstrating the anterior communicating artery aneurysm before treatment (arrow).
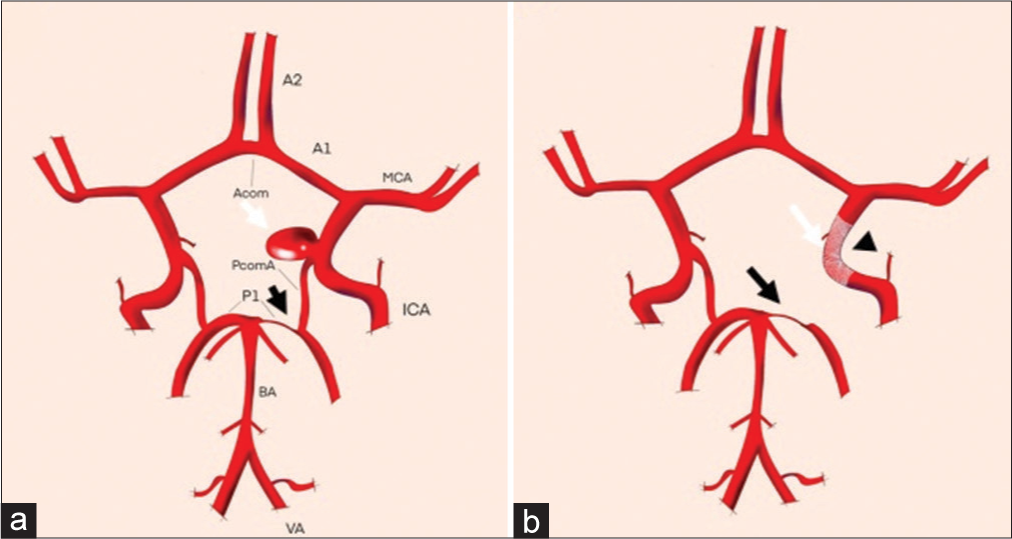
Figure 3:
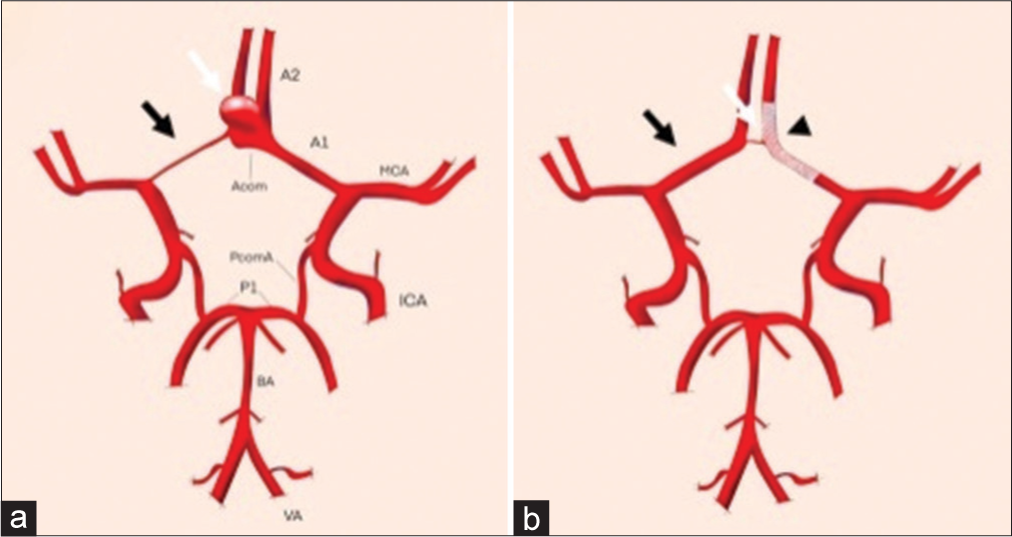
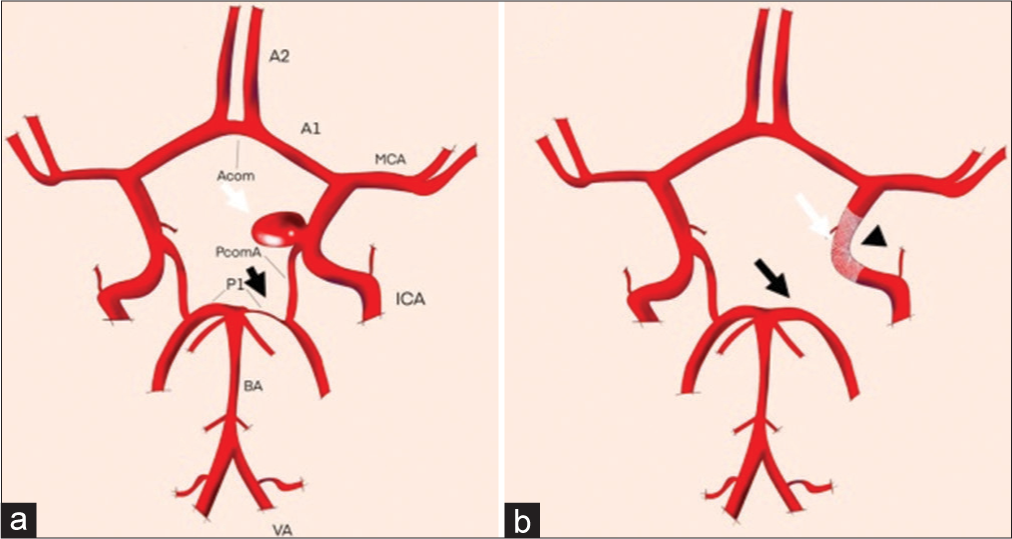
(a) Schematic drawings of patients 1 and 2 showing the anterior communicating artery aneurysm (white arrow) with its neck related to the left A1–A2 anterior cerebral artery and a hypoplastic right A1-anterior cerebral artery (black arrow) (b) Changes at the follow-up represented by the flow diverter stents (FDS) placement in the left A1–A2 with occlusion of the aneurysm sac (white arrow) and increase in the caliber of the right A1-anterior cerebral artery (black arrow) in response to the FDS (arrowhead) effect.
Patient 3
A 61-year-old man with PComA IA was treated with a pipeline flow-diverter implanted in the left ICA, covering the origin of the PComA. In the follow-up angiogram at 6 months, the IA and PComA were occluded. The patient incurred no neurologic deficit which suggests increase flow from the ipsilateral P1-segement of posterior-cerebral-artery (P1-PCA) without change in its caliber by visual angiographic analysis [
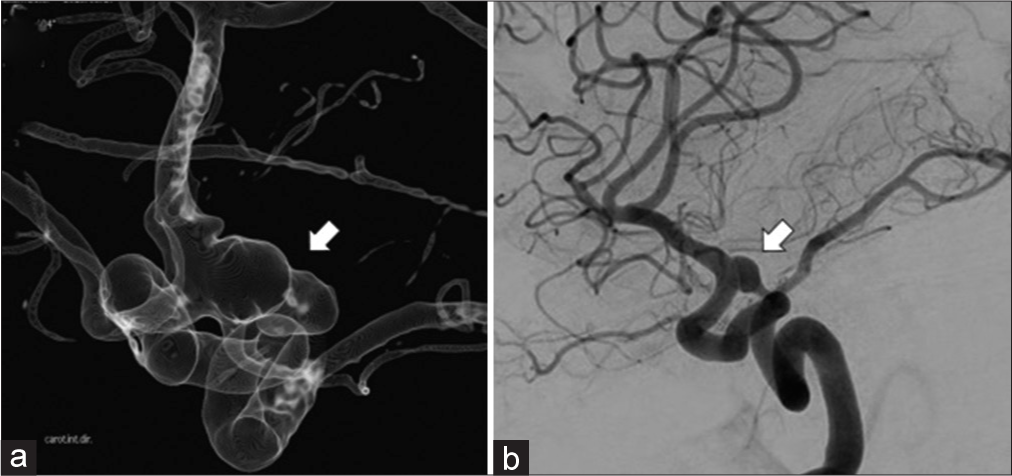
Figure 4:
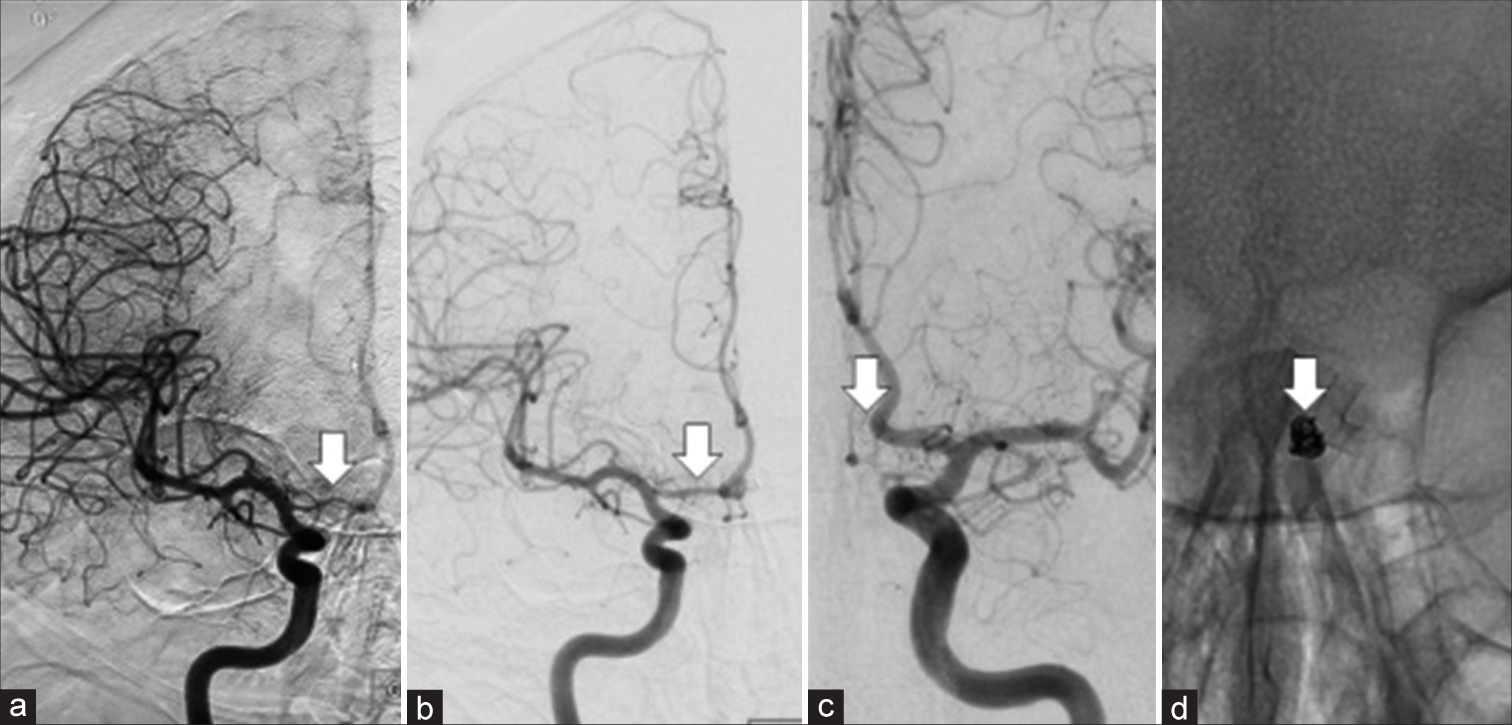
Patient 3 – (a) Coiled aneurysm before flow diverter stents (FDS) placement (arrow) with the posterior communicating artery (PComA) originating from the aneurysm. (b) Follow-up angiogram showing complete occlusion of the aneurysm (arrow) and PComA by the flow diversion effect. (c) Plain X-ray images showing coils and flow diverter stent (arrows). (d) Vertebral angiogram before internal carotid artery FDS placement showing a moderate size P1-segement of posterior-cerebral-artery (arrow) and (e) post FDS with stable size (arrow).
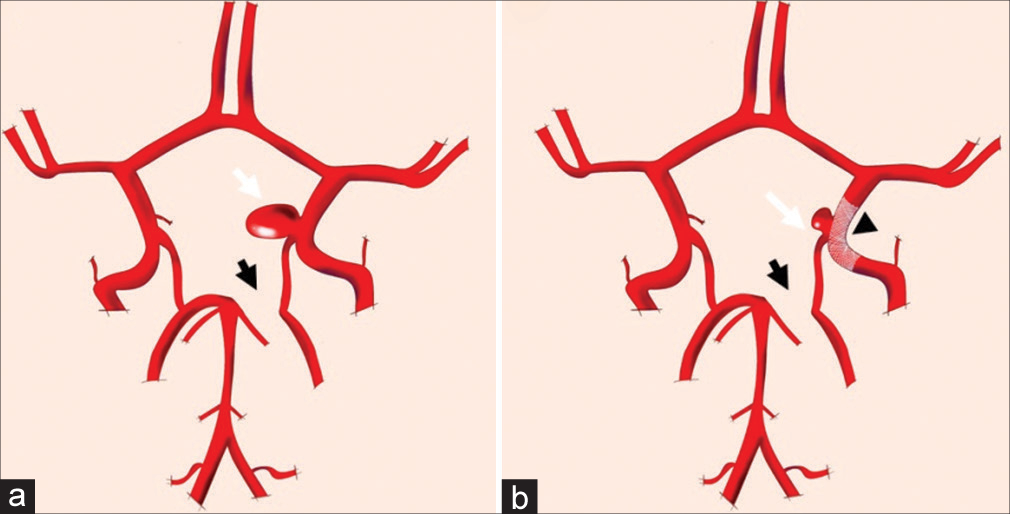
Figure 5:
(a) Schematic drawing of patient 3 showing the posterior communicating artery (PComA) aneurysm (white arrow) and a left hypoplastic P1 (black arrow) before the flow diverter stents (FDS) late effect. (b) After late FDS (arrowhead) effect changes showing occlusion of the aneurysm (white arrow) and adjacent PComA with no significant visual increase in the diameter of the left P1-segement of posterior-cerebral-artery (black arrow).
Patient 4
A 72-year-old woman with IA of PComA and hypoplastic P1-PCA with a fetal type right PCA was treated with a pipeline flow-diverter implanted in the right ICA, across the origin of the PComA. In the follow-up angiogram at 8 months, it was noted that the IA had decreased in size and the fetal PCA remained patent with the same caliber emerging close to the IA neck. The left P1 segment of the left PCA remained hypoplastic with the same caliber. The patient had no neurologic deficits [
Figure 7:
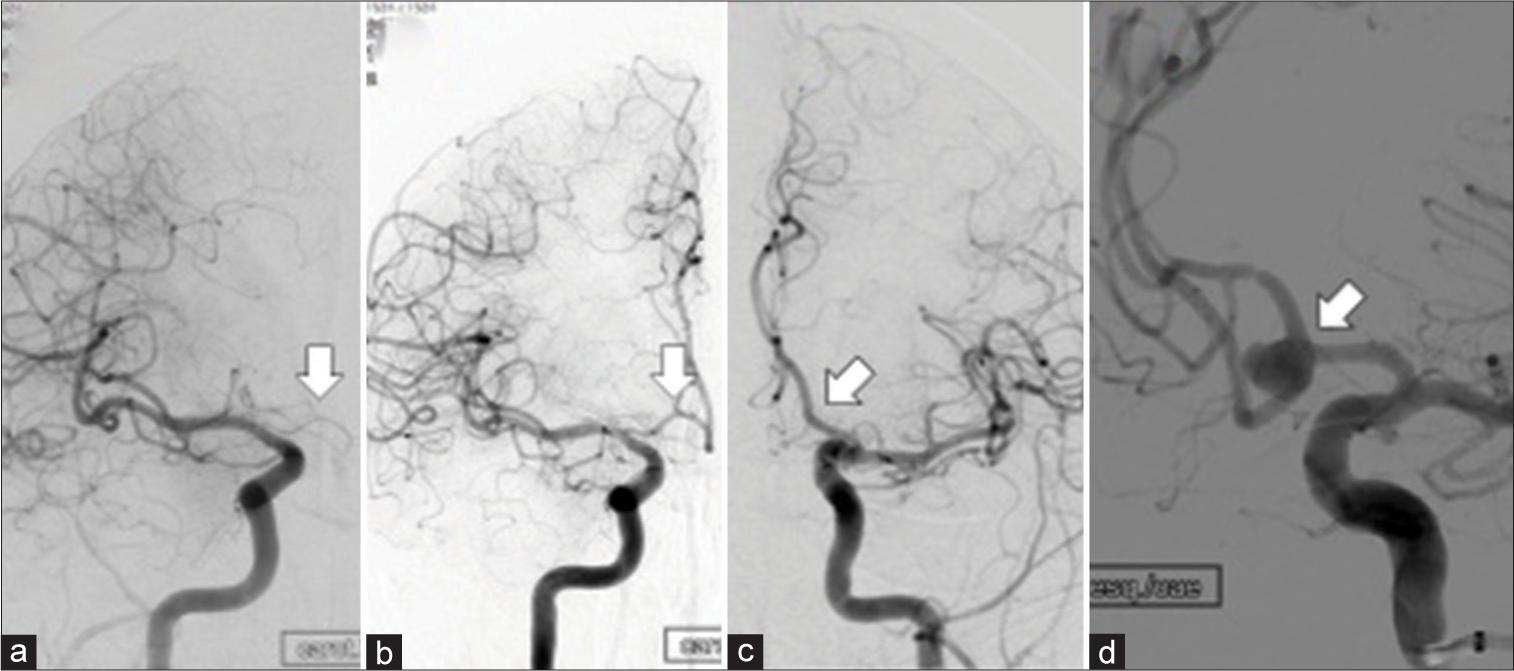
(a) Schematic drawing of patient 4 showing the posterior communicating artery aneurysm with a fetal posterior-cerebral-artery (PCA) arising close to its neck (white arrow) and a left hypoplastic P1 (black arrow) before the late flow diversion effect. (b) After late flow diverter stents (arrowhead) effect changes showing a reduction of the aneurysm size with patency and same size of the left fetal PCA (white arrow) and hypoplastic left P1 (black arrow) at the follow-up.
Patient 5
A 61-year-old woman was found incidentally with a left PComA aneurysm on MRI during work-up for occipital neuralgia. On physical examination, she was neurologically intact. Decision was made to proceed with pipeline stent placement in the ICA across the aneurysm neck. She tolerated the procedure well with complete obliteration of her aneurysm at 2-year follow-up magnetic resonance angiography. However, curiously, in this follow-up examination, the left P1-PCA showed a significant increase in caliber and filling. She remained neurologically intact until her late follow-up at 2 years [
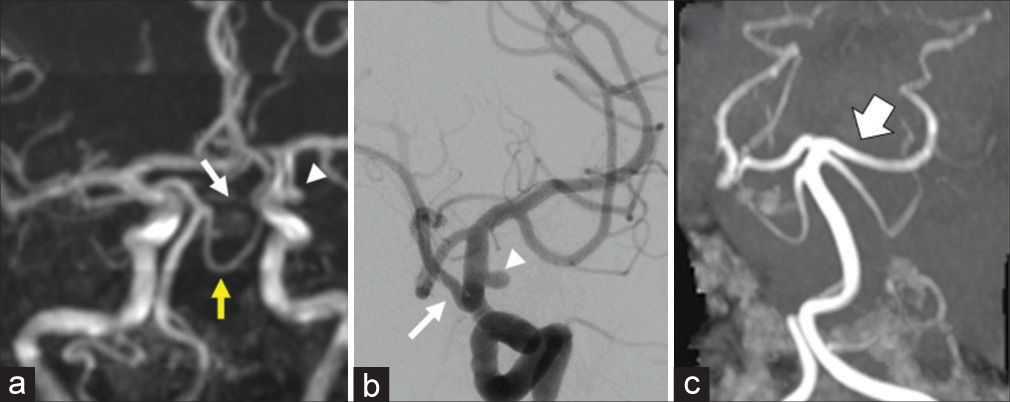
Figure 8:
Patient 5 – (a) MRA showing hypoplastic P1-segement of posterior-cerebral-artery (P1-PCA) (arrow) and the left supraclinoid internal carotid artery (ICA) aneurysm measuring 3.9 mm (arrowhead). Superior cerebellar artery (yellow arrow). (b) Digital subtraction ICA angiogram in oblique view during the endovascular procedure showing the left fetal PCA (arrow) and the aneurysm (arrowhead) covered by the flow diverter stent. (c) Follow-up MRA after ICA FDS placement with increase in the size of the left P1-PCA (arrow) and filling of the ipsilateral PCA.
Figure 9:
(a) Schematic drawing of patient 5 showing the posterior communicating artery (PComA) aneurysm (white arrow) and a left hypoplastic P1 (black arrow) before the flow diverter stents (FDS) late effect. (b) After late FDS (arrowhead) effect changes showing occlusion of the aneurysm (white arrow) and adjacent PComA with significant increase in the diameter of the left P1-segement of posterior-cerebral-artery (black arrow).
DISCUSSION
We have presented five patients that demonstrate different patterns of arterial remodeling of the circle of Willis vessels in adult patient response to flow diversion. These changes can be divided into two main phenomena: (1) in the AComA circulation, a patient with a of hypoplastic or reduced-caliber A1-ACA, can increase the caliber of this vessel in response to having the contralateral ACA segment A1 and A2 covered by the flow diverter. (2) In the posterior circulation, patients with hypoplastic or reduced-caliber P1-PCA or fetal PCA can indeed have increase in the P1-PCA diameter of the vessel after having the origin of the ipsilateral PComA covered by the flow diverter in the ICA and also no neurological deficits after coverage with or without a FDS, leading to occlusion of the ipsilateral PComA.
This phenomenon, which seems to be a compensatory response to the decrease in flow in arteries that emerge from the segment implanted with the flow diverter, is not new in this article, as it has already been reported by other authors.[
In trying to understand more about vascular remodeling responses, two main types of studies have tried to address changes after placement of a flow diverter. Several studies have analyzed the artery adjacent to the device.[
One theory for non-arteries, occlusion of vessels without substantial collaterals, was that in these vessels, the pressure gradient would be greater than in those with collaterals, thus increasing the threshold for occlusion of these vessels. We identify another type of response in the collateral circuits of the circle of Willis. Thus, the lack of substantial collaterals, as in hypoplastic A1-ACA or P1-PCA, in addition to increasing the occlusion threshold of AcomA and PcomA, could also favor the increase in flow and caliber of these previously hypoplastic collaterals vessels. The other two responses are better known phenomena – and include occlusion or reduction of the artery covered by a flow diverter, or the appearance of retrograde pial collaterals in cases of placement of a device in more distal vessels such as the middle cerebral artery bifurcation.
The findings of this study in addition to others raise numerous questions on the subject including the precise hemodynamic mechanisms responsible for remodeling as well as the safety of treating patients with flow dependent on the AComA or PComA with flow diversion. The type of flow diverter used with different mesh density and design may also influence the observed anatomic changes. Future studies that hemodynamically compare these scenarios including use of computational fluid analysis may bring more insight on the subject.
CONCLUSION
The use of flow diverters can affect both vessels “jailed” by the stent and other arteries of the circle of Willis, leading to significant changes in the flow and size of these vessels. These phenomena appear to be a compensatory response to the hemodynamic changes induced by the flow diversion. The issues raised, as well as the safety of treating patients with certain vascular anatomy, require further investigation.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Brinjikji W, Kallmes DF, Cloft HJ, Lanzino G. Patency of the anterior choroidal artery after flow-diversion treatment of internal carotid artery aneurysms. Am J Neuroradiol. 2015. 36: 537-41
2. Brinjikji W, Lanzino G, Cloft HJ, Kallmes DF. Patency of the posterior communicating artery after flow diversion treatment of internal carotid artery aneurysms. Clin Neurol Neurosurg. 2014. 120: 84-8
3. Brouillard AM, Sun X, Siddiqui AH, Lin N. The use of flow diversion for the treatment of intracranial aneurysms: Expansion of indications. Cureus. 2016. 8: e472
4. Cornelissen BM, Schneiders JJ, Sprengers ME, van den Berg R, van Ooij P, Nederveen AJ. Aneurysmal parent artery-specific inflow conditions for complete and incomplete circle of willis configurations. Am J Neuroradiol. 2018. 39: 910-5
5. de Carvalho FM, Caroff J, dos Santos Neto E, Chalumeau V, Khalek HA, Neki H. Flow changes in the posterior communicating artery related to flow-diverter stents in carotid siphon aneurysms. J Neurointerv Surg. 2017. 9: 674-8
6. Gascou G, Lobotesis K, Brunel H, Machi P, Riquelme C, Eker O. Extra-aneurysmal flow modification following pipeline embolization device implantation: Focus on regional branches, perforators, and the parent vessel. Am J Neuroradiol. 2015. 36: 725-31
7. Iosif C, Berg P, Ponsonnard S, Carles P, Saleme S, Ponomarjova S. Role of terminal and anastomotic circulation in the patency of arteries jailed by flow-diverting stents: From hemodynamic changes to ostia surface modifications. J Neurosurg. 2017. 126: 1702-13
8. Litao MS, Burkhardt JK, Tanweer O, Raz E, Huang P, Becske T. Remodeling of the posterior cerebral artery P1-segment after pipeline flow diverter treatment of posterior communicating artery aneurysms. Neurol Int. 2021. 13: 195-201
9. Muneer MS, Todnem N, Gopal N, MarencoHillembrand L, Barakat E, Mbabuike N. Pattern of changes in cross sectional area of arterial branches after jailing with pipeline embolization device: Beyond parent neck artery patency. Clin Imaging. 2022. 83: 159-65
10. Narata AP, de Moura FS, Larrabide I, Perrault CM, Patat F, Bibi R. The role of hemodynamics in intracranial bifurcation arteries after aneurysm treatment with flow-diverter stents. Am J Neuroradiol. 2018. 39: 323-30
11. Patel PD, Chalouhi N, Atallah E, Tjoumakaris S, Hasan D, Zarzour H. Off-label uses of the Pipeline embolization device: A review of the literature. Neurosurg Focus. 2017. 42: E4
12. Puffer RC, Kallmes DF, Cloft HJ, Lanzino G. Patency of the ophthalmic artery after flow diversion treatment of paraclinoid aneurysms. J Neurosurg. 2012. 116: 892-6
13. Ravindran K, Casabella AM, Cebral J, Brinjikji W, Kallmes DF, Kadirvel R. Mechanism of action and biology of flow diverters in the treatment of intracranial aneurysms. Neurosurgery. 2020. 86: S13-9
14. Rouchaud A, Leclerc O, Benayoun Y, Saleme S, Camilleri Y, D’Argento F. Visual outcomes with flow-diverter stents covering the ophthalmic artery for treatment of internal carotid artery aneurysms. Am J Neuroradiol. 2015. 36: 330-6
15. Vedantam A, Rao VY, Shaltoni HM, Mawad ME. Incidence and clinical implications of carotid branch occlusion following treatment of internal carotid artery aneurysms with the pipeline embolization Device. Neurosurgery. 2015. 76: 173-8
16. Wallace AN, Kayan Y, Austin MJ, Almandoz JE, Kamran M, Cross DT. Pipeline embolization of posterior communicating artery aneurysms associated with a fetal origin posterior cerebral artery. Clin Neurol Neurosurg. 2017. 160: 83-7