- Department of Neurosurgery, Avicenne Military Hospital, Marrakech,
- Mohammed V University, Rabat, Morocco.
Correspondence Address:
Ali Akhaddar
Department of Neurosurgery, Avicenne Military Hospital, Marrakech,
Mohammed V University, Rabat, Morocco.
DOI:10.25259/SNI_571_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Akhaddar A. Polyaxial pedicle screw dislocation during screw tightening for posterior spinal lumbar stabilization. Surg Neurol Int 27-Dec-2019;10:255
How to cite this URL: Akhaddar A. Polyaxial pedicle screw dislocation during screw tightening for posterior spinal lumbar stabilization. Surg Neurol Int 27-Dec-2019;10:255. Available from: https://surgicalneurologyint.com/surgicalint-articles/9823/
COMMENTARY
The polyaxial pedicle screw (PAPS) system is widely used for posterior spinal stabilization. Unlike monoaxial screws, the PAPS has the advantage of moving along several different axes, allowing the surgeon some flexibility in their placement. Nevertheless, pedicle screws are associated with their own unique surgical complications.[
CASE SUMMARY
A 21-year-old male, neurologically intact following a motor vehicle accident, had a L3 burst fracture as demonstrated on the emergent spinal computed tomography-scan [
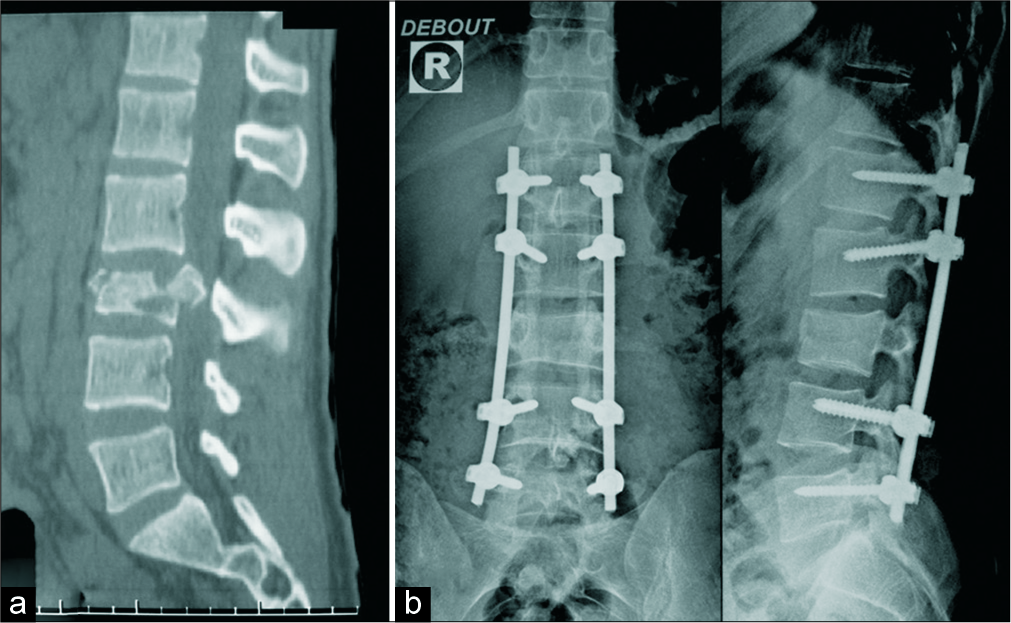
Figure 1:
A burst fracture of L3 as seen on sagittal reconstruction computed tomography scan (bone windows) before operation (a). Postoperative lumbar spinal X-rays showing the emplacement of polyaxial pedicle screws (on L1, L2, L4, and L5), decompressive posterior laminectomy (on L3 and L2) with bilateral connecting rod screws (b).
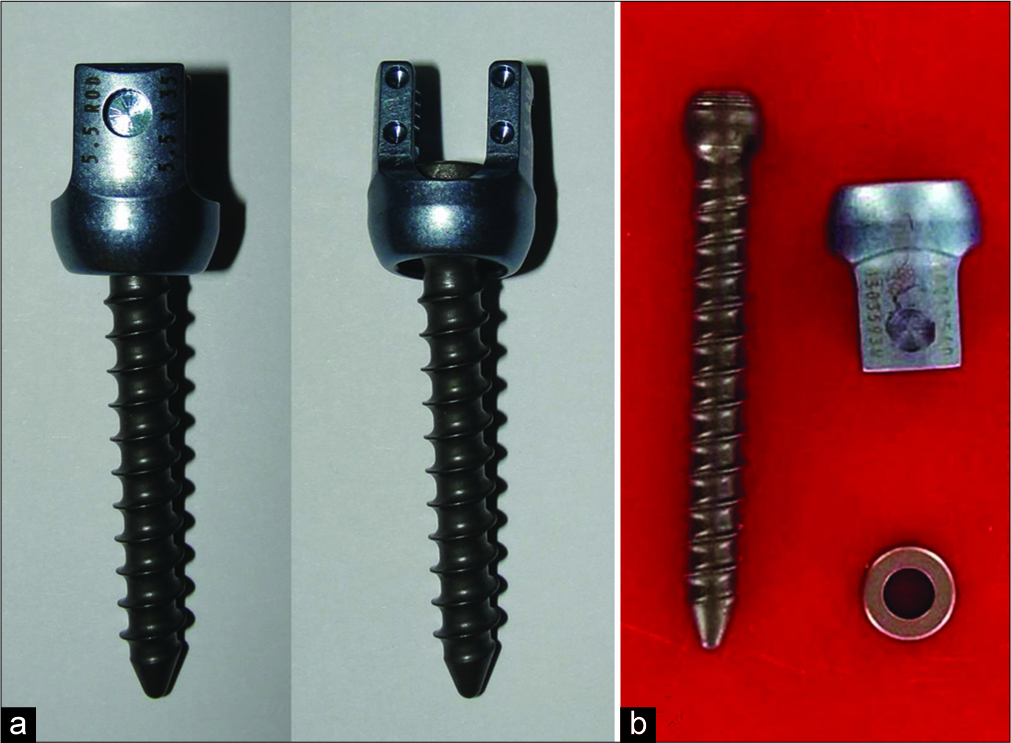
IMPLANT RETURNED TO MANUFACTURER
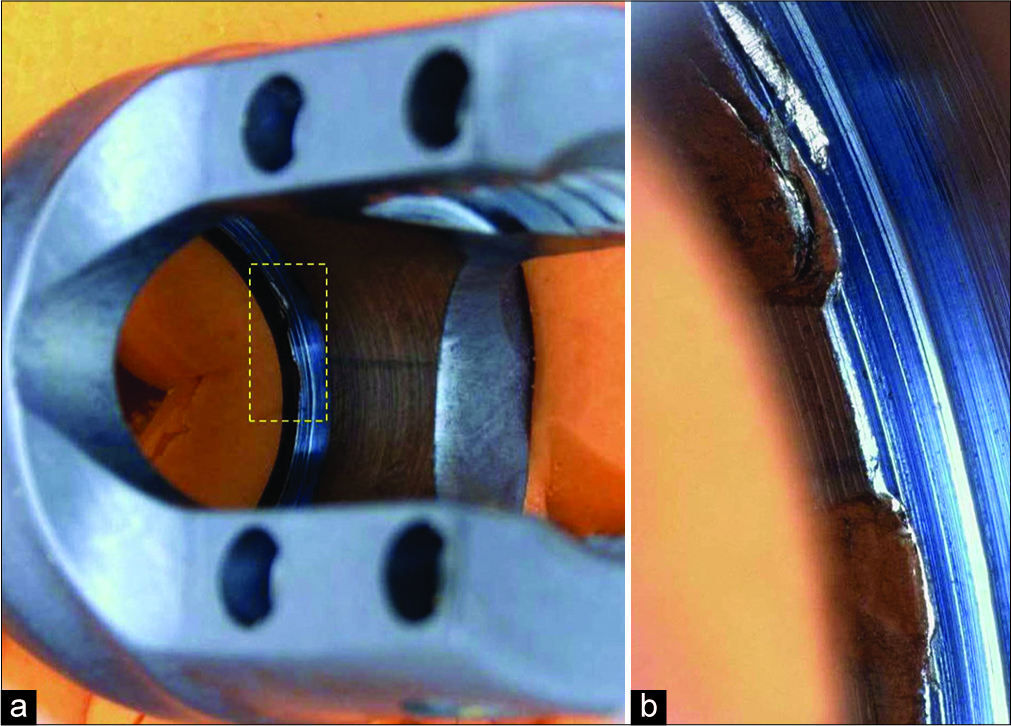
The implant was sent to the manufacturer for their examination; they found “witness marks” and “deformation” noted on the inner diameter surface below the retaining ring pocket of the head; this suggested the ring may not have been fully seated in the ring screw pocket [
This case emphasizes the importance of proper checking of the integrity of the surgical implant before its use. In addition, industry has to guarantee the quality of their product. Here, this unpredictable incident was recognized immediately without serious consequences for the patient. One similar case was previously reported; however, there was no clear manufacturer defect.[
Device component failure should always be considered as a potential risk associated with any spinal system, which may induce neurological/spinal complications and injuries to vessels, nerves, and other organs. Furthermore, incidents and accidents with medical devices must be reported to the National Medicines Agency of each country and the manufacturers to prevent future possible complications.[
Declaration of patient consent
The author certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The author would like to thank the companies Maroc Systèmes Santé (Casablanca, Morocco) and Medtronic Sofamor Danek USA Incorporated for their collaboration to understand this event.
References
1. Akhaddar A, Atmane el M. Pedicle screw malposition following spinal lumbar injury. Pan Afr Med J. 2014. 17: 266-
2. Amoore J, Ingram P. Quality improvement report: Learning from adverse incidents involving medical devices. BMJ. 2002. 325: 272-5
3. Du Plessis PN, Lau BP, Hey HW. Traumatic dislocation of the S1 polyaxial pedicle screw head: A case report. J Spine Surg. 2017. 3: 95-101
4. Gautschi OP, Schatlo B, Schaller K, Tessitore E. Clinically relevant complications related to pedicle screw placement in thoracolumbar surgery and their management: A literature review of 35,630 pedicle screws. Neurosurg Focus. 2011. 31: E8-
5. Lennard N, Coutinho M, Campbell B. The surgeon and medical devices: Adverse incident reporting and off-label use. Ann R Coll Surg Engl. 2013. 95: 309-10






Mario Salmon
Posted February 5, 2020, 8:08 pm
Would be nice to know the brand and manufacturer of those screws in order to prevent further problems. Nowadays there are so many TPS manufacturers that sometimes you never know where they came from.
Elaina Grabow
Posted July 4, 2023, 8:35 am
greetings, honorable blog on oleaginous loss. analogous helped.