- Department of Neurosurgery, Università Politecnica delle Marche, Ancona, Italy
- Department of Neurosurgery, Università Politecnica delle Marche Facoltà di Medicina e Chirurgia, Ancona, Italy
- Department of Neurology, Università Politecnica delle Marche, Ancona, Italy.
Correspondence Address:
Mauro Dobran, Department of Neurosurgery, Università Politecnica delle Marche, Ancona, Italy.
DOI:10.25259/SNI_1090_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mauro Dobran1, Alessandro Di Rienzo2, Erika Carrassi1, Denis Aiudi1, Alessio Raggi1, Alessio Iacoangeli1, Simona Lattanzi3, Maurizio Iacoangeli1. Post-traumatic decompressive craniectomy: Prognostic factors and long-term follow-up. 17-Nov-2023;14:400
How to cite this URL: Mauro Dobran1, Alessandro Di Rienzo2, Erika Carrassi1, Denis Aiudi1, Alessio Raggi1, Alessio Iacoangeli1, Simona Lattanzi3, Maurizio Iacoangeli1. Post-traumatic decompressive craniectomy: Prognostic factors and long-term follow-up. 17-Nov-2023;14:400. Available from: https://surgicalneurologyint.com/surgicalint-articles/12640/
Abstract
Background: Decompressive craniectomy (DC) is still controversial in neurosurgery. According to the most recent trials, DC seems to increase survival in case of refractory intracranial pressure. On the other hand, the risk of postsurgical poor outcomes remain high. The present study aimed to evaluate a series of preoperative factors potentially impacting on long-term follow-up of traumatic brain injury (TBI) patients treated with DC.
Methods: We analyzed the first follow-up year of a series of 75 TBI patients treated with DC at our department in five years (2015–2019). Demographic, clinical, and radiological parameters were retrospectively collected from clinical records. Blood examinations were analyzed to calculate the preoperative neutrophil-to-lymphocyte ratio (NLR). Disability rating scale (DRS) was used to classify patients’ outcomes (good outcome [G.O.] if DRS ≤11 and poor outcome [P.O.] if DRS ≥12) at 6 and 12 months.
Results: At six months follow-up, 25 out of 75 patients had DRS ≤11, while at 12 months, 30 out of 75 patients were included in the G.O. group . Admission Glasgow Coma Scale (GCS) >8 was significantly associated with six months G.O. Increased NLR values and the interval between DC and cranioplasty >3 months were significantly correlated to a P.O. at 6- and 12-month follow-up.
Conclusion: Since DC still represents a controversial therapeutic strategy, selecting parameters to help stratify TBI patients’ potential outcomes is paramount. GCS at admission, the interval between DC and cranioplasty, and preoperative NLR values seem to correlate with the long-term outcome.
Keywords: Cranioplasty, Decompressive craniectomy, Neutrophil-to-lymphocyte ratio, Outcome, Traumatic brain injury
INTRODUCTION
According to the most recent clinical trials,[
Our study aimed to reconsider the long-term follow-up of TBI patients treated by DC over five years at our institution, thoroughly analyzing a series of preoperative clinical, radiological, and hematological factors potentially impacting patients’ prognosis with the aim of a successful case selection.
MATERIALS AND METHODS
We analyzed retrospectively the first follow-up year of a series of 75 TBI patients treated with DC and subsequent cranioplasty at our department from January 2015 to December 2019.
Gender, age, comorbidities (hypertension, diabetes mellitus [DM], and use of antiplatelet drug), Glasgow Coma Scale (GCS), and Rotterdam Scores (RS) were assessed in all patients at admission; full blood count was also obtained to establish the neutrophil-to-lymphocyte ratio (NLR). Clinical outcomes at 6 and 12 months were evaluated by the disability rating scale (DRS) through direct or telephonic interviews with the patients or their family members. Based on their final DRS, subjects were classified into two main classes, respectively, “good outcome group” (G.O., no or moderate disability, [DRS 0-11]) and “poor outcome group” (P.O., severe disability or dead [DRS 12–30]). Finally, the interval time between craniectomy and cranioplasty was also considered. For each patient, the follow-up time after cranioplasty was 18 months. Nontraumatic patients, subjects <16 years of age or with previous craniotomy, known compromised cognitive and motor performance subsequent to previous neurological damage from different causes were excluded from the study.
Data analysis was performed using the STATA/IC 13.1 statistical package (StataCorp LP, Texas, USA). Values are presented as the median (interquartile range) for continuous variables and the number (percent) of subjects for categorical variables. Univariate comparison was made through the Mann–Whitney test or Chi-squared test, as appropriate. The association between baseline characteristics and 6- and 12-month functional outcomes was determined using logistic regression analysis. Results were considered significant for P < 0.05 (two-sided).
RESULTS
Over the considered period, given the exclusion criteria, 75 TBI patients were treated with DC over the considered period, of which 62 men (82.7%) and 13 women (17.3%) were enrolled in the current study.
Comorbidities were distributed as follows: 27 patients (36%) suffered from hypertension, 33 patients (44%) from DM, and 15 patients (20%) were on antiplatelet drugs.
The findings identified on computed tomography (CT) performed on arrival at the emergency department were as follows: 41% of patients presented with acute subdural hematoma, 35% with hemorrhagic contusions, 14% with epidural hematoma, and 10% with diffuse cerebral edema.
At six months follow-up, the G.O. consisted of 25 out of 75 patients, while at 12 months follow-up, the same group was composed of 30 out of 75 subjects.
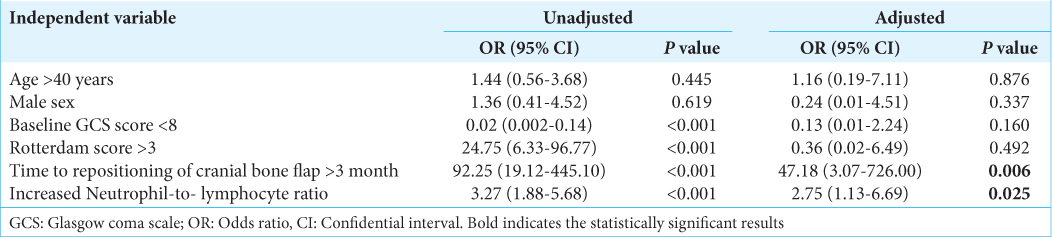
The data analyzed and compared between patients with G.O. and P.O. at 6 and 12 months are illustrated in
Neither age nor gender resulted significantly in statistical analysis.
Admission GCS <8 was a significant predictor of P.O. both at 6- and at 12-month follow-up at the univariate analysis. However, this finding maintained its statistical significance in the multivariate analysis only at 6-month follow-up. (P = 0.018).
Although high RS resulted in a significant predictor of P.O. at the univariate analysis at 6 and 12 months follow-up, the multivariate analysis did not confirm its statistical significance.
A time interval >3 months between the DC and the bone flap repositioning resulted in a statistically significant parameter associated with P.O. at 6- and 12-month follow-up (6 months P = 0.032; 12 months P = 0.006).
The NLR was significantly higher in P.O. patients than G.O.; in particular, mean NLR was, respectively, 3.95 (range, 2.65–4.91) and 1.86 (range, 1.22–2.56) at 6-month follow-up and 4.10 (range, 2.87–4.96) and 1.86 (range, 1.22–2.58) at 12-month follow-up. The statistical significance of this finding was confirmed at multivariate analysis (6 months P = 0.04; 12 months P = 0.003).
DISCUSSION
Indications to DC for intractable ICP remain a controversial matter. Technical aspects, timing of surgery, and patient selection are continuously debated in the neurosurgical community.[
According to the most recent Brain Trauma Foundation guidelines,[
Conventionally, clinical parameters such as age, admission GCS, pupillary response, and radiological assessment have helped neurosurgeons to guide the decision.[
Sahuquillo and Dennis’ Cochrane Review[
In our series, the male sex was predominant, constituting 82.7% of the whole population sample and corroborating the results of the epidemiologic studies on TBI.[
Although age is a well-known TBI prognostic parameter,[
The evaluation of the admission GCS score is paramount to predicting the potential neurological result of a surgical procedure.[
A possible bias in our study could be the delay in surgery due to the transport time from remote areas. After admission to our hospital, that is the only level III trauma center in the region, there was no delay in surgery timing.
The Rotterdam Scoring System helps to estimate the posttrauma 6-month prognosis and mortality using radiological criteria.[
In our series, an RS >3 was found in the 70% and the 73.3% of P.O. patients, respectively, at 6 and 12 months follow-up. This was largely expected since RS increases with the severity of the radiological findings. Nevertheless, the statistical significance of this parameter as an independent prognostic factor for TBI was not confirmed at the multivariate analysis both at six and at 12 months follow-up.
Cranioplasty is another important issue related to DC: the correct timing to perform it, the selection of the most suitable material to reduce/avoid complications (e.g., infections, seizures, bone flap resorption, hydrocephalus, hemorrhage, and cosmetic issues), the storage/fixation techniques are still controversial.[
The optimal timing of cranioplasty is still ill-defined: some authors[
In the present study, cranioplasty performed after three or more months from DC result associated with P.O. at 6- and 12-month follow-up.
This finding could be explained by the assumption that the earlier the cranioplasty, the earlier the restoration of some potential abnormalities caused by the DC (e.g., cerebrospinal fluid dynamics disturbances, altered cerebral perfusion, and metabolic rate of oxygen and glucose).[
Moreover, cranioplasty can help to prevent/solve eventual syndromes which may complicate the postoperative recovery after DC (e.g., Syndrome of the trephined and craniectomy-associated progressive extra-axial collections with treated hydrocephalus).[
Recently, researchers have focused on the prognostic role of the systemic inflammatory response in neurological diseases and trauma, with the intent of increasing the accuracy of the already existing prognostic models such as CRASH and IMPACT.[
NLR is now a worldwide accepted significant index of inflammation, and its value in predicting the outcome after a TBI is widely discussed in the literature. Its advantages over the other prognostic factors lie in the ease of obtaining, the low cost, and the objectivity of the datum.
Over the past years, several studies have suggested a correlation between the increase in admission NLR and the P.O. of various diseases, including TBI.[
Chen et al.[
Siwicka-Gieroba et al. [
Zhao et al.[
On the contrary, Corbett et al.[
A recent review conducted by Sabouri et al.[
In this complex picture, the results of our study seem to corroborate the hypothesis that admission NLR value could help to stratify the long-term outcome in TBI patients. In fact, in the P.O., the mean admission NLR was higher than in the G.O. This difference was significant in both the univariate and multivariate analysis.
However, it is important to underline that the values found in our investigation were not particularly high in both groups, even if the mean NLR exceeded the healthy adults cutoff value (3.53)[
Nonetheless, the NLR values found in our study are much lower than those indicated by Chen et al. (13.05)[
CONCLUSION
The selection of parameters that can help stratify TBI patients’ potential outcomes is paramount. In our study, the GCS, the timing of the cranioplasty, and the admission NLR resulted in independent predictor factors associated with the 6-month outcome, while only the timing of the cranioplasty and the NLR significantly correlated with the 12-month outcome of patients treated with DC due to a TBI.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Our Local Ethics Committee waived ethical approval in view of the study’s retrospective nature, and all the procedures being performed were part of the routine care.
Data availability
Patients and their families were assured that data would remain confidential and would not be shared.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alkhaibary A, Alharbi A, Alnefaie N, Oqalaa Almubarak A, Aloraidi A, Khairy S. Cranioplasty: A comprehensive review of the history, materials, surgical aspects, and complications. World Neurosurg. 2020. 139: 445-52
2. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: An overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020. 104: 213-38
3. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010. 112: 1120-4
4. Charry JD, Navarro-Parra S, Solano J, Moscote-Salazar L, Pinzón MA, Tejada JH. Outcomes of traumatic brain injury: The prognostic accuracy of various scores and models. Neurol Neurochir Pol. 2019. 53: 55-60
5. Chen J, Qu X, Li Z, Zhang D, Hou L. Peak neutrophil-tolymphocyte ratio correlates with clinical outcomes in patients with severe traumatic brain injury. Neurocrit Care. 2019. 30: 334-9
6. Chen W, Yang J, Li B, Peng G, Li T, Li L. Neutrophil to lymphocyte ratio as a novel predictor of outcome in patients with severe traumatic brain injury. J Head Trauma Rehabil. 2018. 33: E53-e9
7. Chester AN, Purdie GL, Dennett ER, Parker AJ. A survey of neurosurgical management and prognostication of traumatic brain injury following the RESCUEicp trial. Br J Neurosurg. 2021. 35: 329-33
8. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011. 364: 1493-502
9. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, Ponsford J. Patient outcomes at twelve months after early decompressive craniectomy for diffuse traumatic brain injury in the randomized DECRA clinical trial. J Neurotrauma. 2020. 37: 810-6
10. Corallo F, De Cola MC, Lo Buono V, Marra A, De Luca R, Trinchera A. Early vs late cranioplasty: What is better?. Int J Neurosci. 2017. 127: 688-93
11. Corbett JM, Ho KM, Honeybul S. Prognostic significance of abnormal hematological parameters in severe traumatic brain injury requiring decompressive craniectomy. J Neurosurg. 2019. 132: 545-51
12. Dhandapani S, Manju D, Sharma B, Mahapatra A. Prognostic significance of age in traumatic brain injury. J Neurosci Rural Pract. 2012. 3: 131-5
13. Dobran M, Nasi D, Polonara G, Paracino R, Mancini F, Costanza MD. Clinical and radiological risk factors of autograft cranioplasty resorption after decompressive craniectomy for traumatic brain injury. Clin Neurol Neurosurg. 2020. 196: 105979
14. Dolmans RG, Hulsbergen AF, Gormley WB, Broekman ML. Routine blood tests for severe traumatic brain injury: Can they predict outcomes?. World Neurosurg. 2020. 136: e60-7
15. Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio?. BMC Res Notes. 2017. 10: 12
16. Goedemans T, Verbaan D, van der Veer O, Bot M, Post R, Hoogmoed J. Complications in cranioplasty after decompressive craniectomy: Timing of the intervention. J Neurol. 2020. 267: 1312-20
17. Gregson BA, Rowan EN, Francis R, McNamee P, Boyers D, Mitchell P. Surgical trial in traumatic intracerebral haemorrhage (STITCH): A randomised controlled trial of Early Surgery compared with Initial Conservative Treatment. Health Technol Assess. 2015. 19: 1-138
18. Gupte R, Brooks W, Vukas R, Pierce J, Harris J. Sex differences in traumatic brain injury: What we know and what we should know. J Neurotrauma. 2019. 36: 3063-91
19. Hawryluk GW, Rubiano AM, Totten AM, O’Reilly C, Ullman JS, Bratton SL. Guidelines for the management of severe traumatic brain injury: 2020 Update of the decompressive craniectomy recommendations. Neurosurgery. 2020. 87: 427-34
20. Honeybul S, Ho KM, Lind CR, Gillett GR. Validation of the CRASH model in the prediction of 18-month mortality and unfavorable outcome in severe traumatic brain injury requiring decompressive craniectomy. J Neurosurg. 2014. 120: 1131-7
21. Honeybul S, Ho KM. Predicting long-term neurological outcomes after severe traumatic brain injury requiring decompressive craniectomy: A comparison of the CRASH and IMPACT prognostic models. Injury. 2016. 47: 1886-92
22. Huang YH, Deng YH, Lee TC, Chen WF. Rotterdam computed tomography score as a prognosticator in head-injured patients undergoing decompressive craniectomy. Neurosurgery. 2012. 71: 80-5
23. Hutchinson PJ, Kolias AG, Tajsic T, Adeleye A, Aklilu AT, Apriawan T. Consensus statement from the international consensus meeting on the role of decompressive craniectomy in the management of traumatic brain injury: Consensus statement. Acta Neurochir (Wien). 2019. 161: 1261-74
24. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016. 375: 1119-30
25. Kim BW, Kim TU, Hyun JK. Effects of early cranioplasty on the restoration of cognitive and functional impairments. Ann Rehabil Med. 2017. 41: 354-61
26. Kreitzer N, Rath K, Kurowski BG, Bakas T, Hart K, Lindsell CJ. Rehabilitation practices in patients with moderate and severe traumatic brain injury. J Head Trauma Rehabil. 2019. 34: E66-72
27. Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018. 387: 98-102
28. Lee SY, Amatya B, Judson R, Truesdale M, Reinhardt JD, Uddin T. Clinical practice guidelines for rehabilitation in traumatic brain injury: A critical appraisal. Brain Inj. 2019. 33: 1263-71
29. Malcolm JG, Rindler RS, Chu JK, Chokshi F, Grossberg JA, Pradilla G. Early cranioplasty is associated with greater neurological improvement: A systematic review and meta-analysis. Neurosurgery. 2018. 82: 278-88
30. Perel P, Arango M, Clayton T, Edwards P, Komolafe E. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ. 2008. 336: 425-9
31. Nasi D, Gladi M, Di Rienzo A, di Somma L, Moriconi E, Iacoangeli M. Risk factors for post-traumatic hydrocephalus following decompressive craniectomy. Acta Neurochir (Wien). 2018. 160: 1691-8
32. National database: 2017 profile of people within the traumatic brain injury model systems. Traumatic brain injury model systems National Data and Statistical Center. Available from: https://msktc.org/lib/docs/data_sheets_/2017_tbims_national_database_update_1.pdf [Last accessed on 2023 Oct 12].
33. Posti JP, Yli-Olli M, Heiskanen L, Aitasalo KJ, Rinne J, Vuorinen V. Cranioplasty after severe traumatic brain injury: Effects of trauma and patient recovery on cranioplasty outcome. Front Neurol. 2018. 9: 223
34. Sabouri E, Majdi A, Jangjui P, Rahigh Aghsan S, Naseri Alavi SA. Neutrophil-to-lymphocyte ratio and traumatic brain injury: A review study. World Neurosurg. 2020. 140: 142-7
35. Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev. 2019. 12: CD003983
36. Schuss P, Vatter H, Marquardt G, Imöhl L, Ulrich CT, Seifert V. Cranioplasty after decompressive craniectomy: The effect of timing on postoperative complications. J Neurotrauma. 2012. 29: 1090-5
37. Siwicka-Gieroba D, Malodobry K, Biernawska J, Robba C, Bohatyrewicz R, Rola R. The neutrophil/lymphocyte count ratio predicts mortality in severe traumatic brain injury patients. J Clin Med. 2019. 8: 1453
38. Wang Z, Gong Q, Guo C, Luo Y, Chen L. Neutrophil-tolymphocyte ratio predicts hematoma growth in intracerebral hemorrhage. J Int Med Res. 2019. 47: 2970-5
39. Zhao JL, Du ZY, Yuan Q, Yu J, Sun YR, Wu X. Prognostic value of neutrophil-to-lymphocyte ratio in predicting the 6-month outcome of patients with traumatic brain injury: A retrospective study. World Neurosurg. 2019. 124: e411-6
40. Zheng F, Xu H, von Spreckelsen N, Stavrinou P, Timmer M, Goldbrunner R. Early or late cranioplasty following decompressive craniotomy for traumatic brain injury: A systematic review and meta-analysis. J Int Med Res. 2018. 46: 2503-12