- Division of Pediatric Neurosurgery, Hospital Provincial Neuquen Dr. Castro Rendon, Neuquen, Argentina.
- Department of Pediatrics, Grupo CMIC, Neuquen, Argentina.
Correspondence Address:
Juan Pablo Mengide, Division of Pediatric Neurosurgery, Hospital Provincial Neuquen Dr. Castro Rendon, Neuquen, Argentina.
DOI:10.25259/SNI_43_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Juan Pablo Mengide1, María Florencia Berros2, Mariana Estefanía Turza2, Juan Manuel Liñares1. Posterior fossa tumors in children: An update and new concepts. 31-Mar-2023;14:114
How to cite this URL: Juan Pablo Mengide1, María Florencia Berros2, Mariana Estefanía Turza2, Juan Manuel Liñares1. Posterior fossa tumors in children: An update and new concepts. 31-Mar-2023;14:114. Available from: https://surgicalneurologyint.com/surgicalint-articles/12228/
Abstract
Background: Posterior fossa tumors account for approximately half of the central nervous system tumors in children. Major technological advances, mainly in the fields of molecular biology and neuroimaging, have modified their classification, leading to a more detailed description of these entities. Into the classic taxonomy, used for many years, new concepts have been incorporated at times eliminating or modifying former ones.
Methods: A literature search was conducted in PubMed using the medical subject headings involving the five most common pediatric posterior fossa tumors: diffuse midline glioma, medulloblastoma, ependymoma, atypical teratoid/rhabdoid tumor, and pilocytic astrocytoma. Only English published articles in the past 11 years that provided technological, neuroimaging, and molecular biology insight into posterior fossa tumors in children were considered.
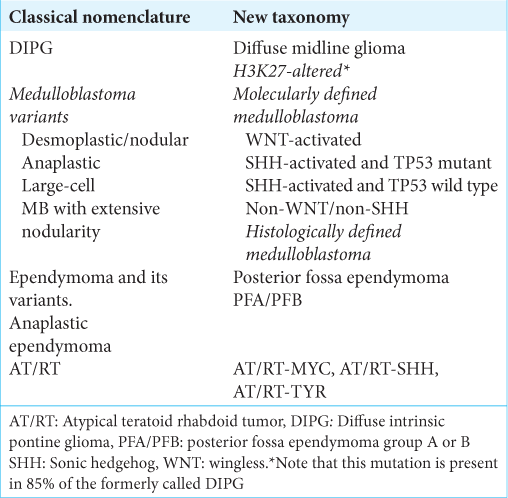
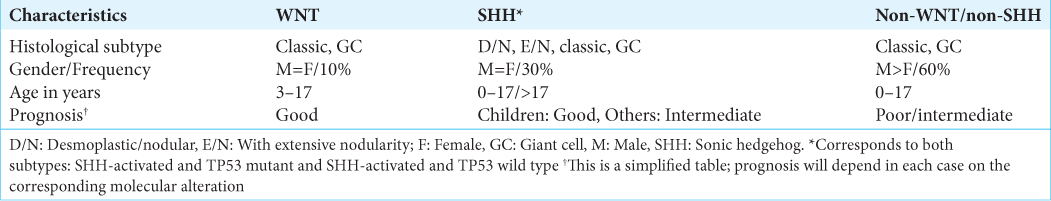
Results: Substantial changes have been introduced in the nomenclature of pediatric posterior fossa tumors. Diffuse midline gliomas are named based on alterations in histone H3. Molecular rearrangements of medulloblastomas are more important in defining the prognosis than histological variants; therefore, these tumors are currently named based on their molecular subgroups. Posterior fossa ependymomas and atypical teratoid rhabdoid tumor classification have incorporated new groups based on different genetic profiles. Pilocytic astrocytoma has been placed in a new category that distinguishes circumscribed from diffuse entities.
Conclusion: Advances in molecular biology and neuroimaging have substantially changed the way pediatric neoplasms are studied. The classical taxonomy has been modified leading to more accurate classifications that are based on the genetic alterations.
Keywords: Pediatrics, Posterior fossa, Tumor
INTRODUCTION
The posterior fossa is the area of the brain that extends from the tentorium to the foramen magnum and contains the most complex brain structures that regulate vital functions.[
In recent years, there have been great technological advances in molecular biology and neuroimaging, which have extended our knowledge of these entities, thereby improving associated morbidity and mortality rates.[
In this review, we provide an update and describe new concepts of the five most common posterior fossa tumors in pediatrics: diffuse midline glioma, medulloblastoma, ependymoma, atypical rhabdoid teratoid tumor, and pilocytic astrocytoma.
MATERIALS AND METHODS
A literature search was conducted in PubMed using the medical subject headings (MESH): adolescent, brain neoplasms/diagnostic imaging, brain neoplasms/ epidemiology, brain neoplasms/pathology, cerebellar neoplasms/epidemiology, cerebellar neoplasms/pathology, ependymoma/pathology, glioma/epidemiology, glioma/ pathology, infant, magnetic resonance imaging (MRI)/ methods, medulloblastoma/pathology, newborn, rhabdoid tumor/epidemiology, and rhabdoid tumor (RT)/pathology. Articles published in the past 11 years that provided technological, neuroimaging, and molecular biology insight into posterior fossa tumors in children were considered. Only English published articles describing diffuse midline glioma, medulloblastoma, ependimoma, atypical teratoid rhabdoid tumor (AT/RT), and pilocytic astrocytoma were reviewed. Other posterior fossa tumors (hemangioblastoma, familial cancer syndromes, arachnoid cysts, epidermoid/dermoid cysts, etc.) were excluded from the study.
RESULTS
General concepts
The clinical presentation of posterior fossa neoplasms is variable. Onset may be acute, subacute, or chronic with cognitive or behavioral disturbances as well as neurological or gastrointestinal manifestations.[
Whole brain and spine MRI are currently a mandatory study for the presurgical planning and staging of these patients and multiple techniques of broad clinical utility may be used [
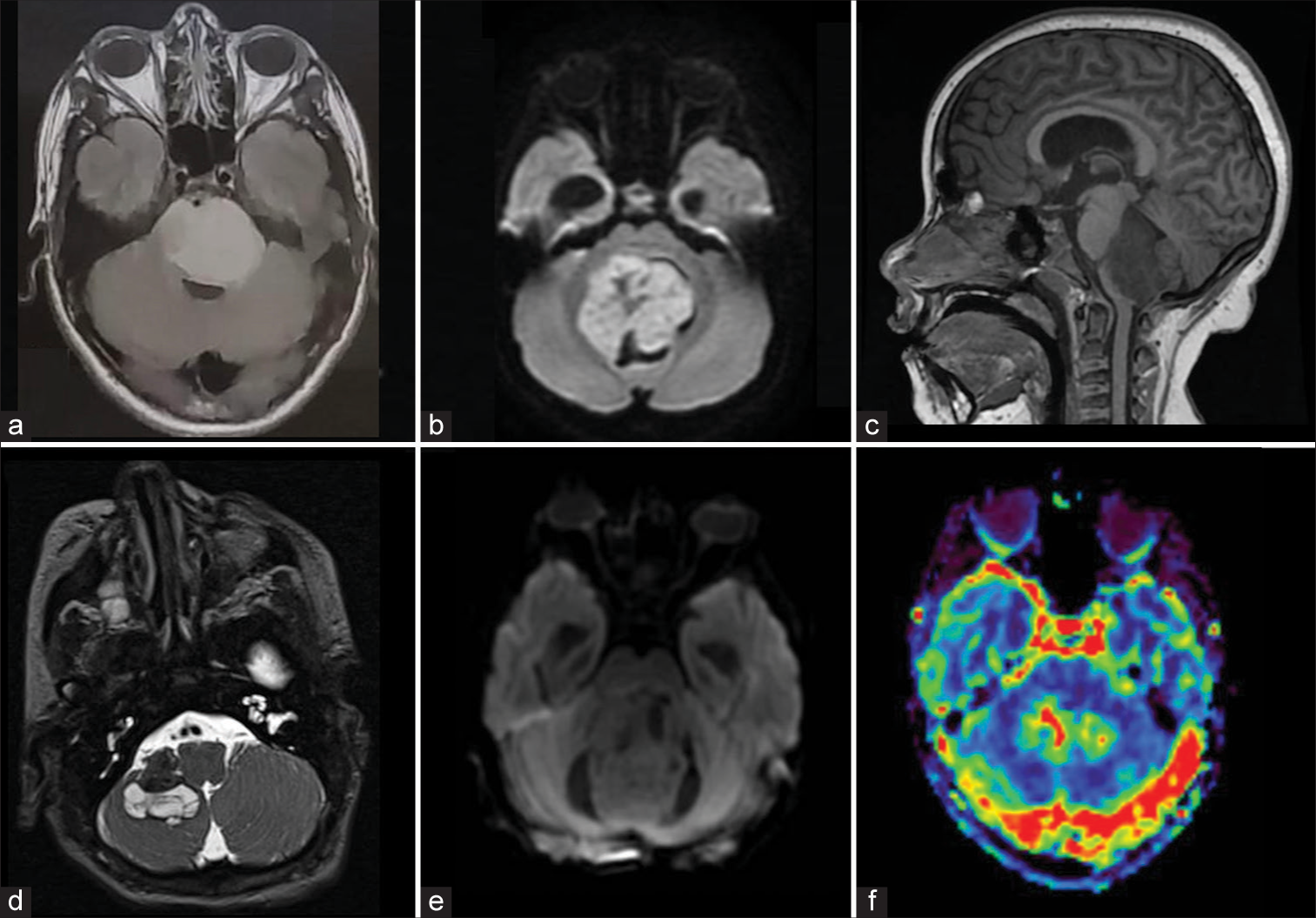
Figure 1:
Magnetic resonance imaging showing some of the hallmark characteristics of posterior fossa tumors. (a) Axial fluid-attenuated inversion recovery sequence image showing the distorted anatomy of a diffuse midline glioma H3 K27-altered enlarging the pons, collapsing the fourth ventricle, and displacing the basilar artery, (b) Axial diffusion-weighted image of a medulloblastoma showing high signal intensity that is characteristic of this World Health Organization grade 4 hypercellular tumors, (c) Sagittal T1-weighted image showing a midline lesion involving the floor of the fourth ventricle due to a group B posterior fossa ependymoma, (d) Axial T2-weighted image of an atypical teratoid rhabdoid tumor showing hemorrhagic areas with low signal intensity and cystic regions of variable signal intensity, (e) Axial diffusion-weighted image of a pilocytic astrocytoma. Note the lower signal intensity in this tumor compared to image b; (f) Perfusion-weighted image of a highly vascularized medulloblastoma showing an area of increased cerebral blood volume.
T1-weighted MRI sequences are the classic technique to evaluate the anatomy of the CNS and contrast administration provides information on the blood-brain barrier permeability. Disruption or integrity of the latter provides insight into the possible tumor type. T2 and fluid-attenuated inversion recovery techniques can show tumor vascularization, edema, and white matter lesions.[ Diffusion-weighted imaging (DWI) uses movement of water molecules to assess tumor cellularity and tissue swelling, showing restrictive diffusion in the above-described conditions. When combined with the previous mentioned techniques, DWI is useful to differentiate higher grade tumors including medulloblastoma and ependymoma from lower grade tumors such as pilocytic astrocytoma.[ MRI spectroscopy is useful to investigate the metabolic profile of the tumor, that is, it provides information on the concentration of certain metabolites (choline, taurine, lactate, etc.) in the tumor, providing information on tumor grade and possibly type as well as the presence of necrosis.[ Susceptibility-weighted imaging is used to evaluate the presence of blood and calcium. The technique assesses vascularity as well as presence of hemoglobin molecules and intratumoral calcifications, and has, in certain contexts, replaced the computed tomography (CT) scan for the identification of calcium in the tumor. Noteworthy, there are some surgical scenarios where the presence of calcium during the presurgical planning is better assessed using CT scan. Perfusion techniques compare tumor blood volume and blood flow to that of normal brain parenchyma and are used to evaluate the possible tumor grade, recurrence, or tumor type. They may be used with or without contrast-enhancement.[ Finally, tractography is used to evaluate the location of the tumor regarding the white matter tracts providing the surgeon with important information to avoid white matter-related postoperative impairments. Functional MRI is used to localize eloquent areas of the brain mainly in the setting of supratentorial tumors; however, these are beyond the focus of this study.
The techniques described above, when interpreted by an expert neuroradiologist, allow for the preoperative prediction of the histology and the altered molecular pathway of the tumor. These new MRI techniques may reveal particular radiological patterns that suggest specific biological behavior of the tumor and can be useful to determine certain genetic alterations, a concept that is currently called “radiogenomics.”[
The main treatment of posterior fossa tumors consists of surgery, chemotherapy, and radiotherapy. At present, molecularly targeted approaches have been described.[
Below we provide an update of the five most common posterior fossa tumors in pediatrics according to the fifth edition of the WHO classification of tumors of the CNS.
Diffuse midline glioma
Before the 2016 WHO classification of tumors of the CNS, the most common pediatric brainstem tumor was termed diffuse intrinsic pontine glioma or DIPG as it is usually called on the hospital ward. DIPG arises in the pons of the brainstem infiltrating it locally and is not amenable to surgical resection [
Medulloblastoma
Medulloblastoma is the most common malignant brain tumor in children, affecting mostly males with a bimodal peak of incidence at 3 years and at 9 years of age [
This new approach prioritizes genetic changes and emphasizes the importance of the molecular biology of medulloblastoma in defining the prognosis. In other words, it is considered that not the histological variant, but the molecular alteration of medulloblastoma mainly defines the behavior of the tumor.[
Ependymoma
Ependymoma is the third most common tumor of the posterior fossa and mainly arises from the floor of the fourth ventricle.[
AT/RT
AT/RT is a highly vascularized and aggressive neoplasm that mostly affects children under 2 years of age [
Pilocytic astrocytoma
Pilocytic astrocytoma is the most common low-grade tumor in children [
Other observations
Certain modifications in the general nomenclature introduced by the 2021 WHO classification of tumors of the CNS have an impact on how we approach the posterior fossa tumors described in this study. In addition, there are many others, such as the extensive modifications in the taxonomy of supratentorial tumors, that do not directly affect them. The following is a summary of the most relevant changes that are directly or indirectly associated with the lesions discussed here.
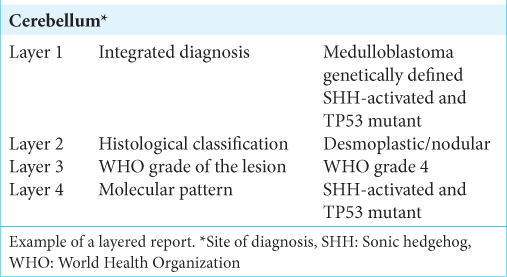
First, advances in the field of molecular biology and its role in the outcome of these diseases made it necessary to incorporate these data into the classification and pathology reports. The new approach to the description of tumors of the CNS is through the concept of an integrated diagnosis composed of different layers.[
Two new suffixes have been added to describe different clinical scenarios. The suffix not otherwise specified (NOS) is used when histopathological or molecular data are not sufficient to make the diagnosis. If the corresponding molecular and histological analyses have been performed, but do not lead to a diagnosis based on the WHO classification, the suffix not elsewhere classified (NEC) is added.[
On the other hand, in the 2021 WHO classification, pediatric gliomas are separated from adult gliomas, resulting in four groups for gliomas: adult diffuse gliomas, pediatric-type diffuse low-grade gliomas, pediatric-type diffuse high-grade gliomas, and circumscribed gliomas (of which the latter includes pilocytic astrocytoma as described above).[
Finally, for a better understanding and to avoid confusion, the Roman numerals to denote tumor grade have been replaced by Arabic numerals and a lesion previously named “Grade IV tumor” is now termed “Grade 4 tumor” [
CONCLUSION
Advances in molecular biology and neuroimaging have substantially changed the way pediatric neoplasms are studied, improving the understanding of posterior fossa tumors and leading to more accurate classifications. The classical taxonomy has been modified and the new terms based on the genetic alterations of tumors allow for a better understanding and study of tumors. Undoubtedly, this new taxonomy will lead to better diagnosis and treatment and improvement in the quality of life of children with brain tumors.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We thank Janneke Deurloo for providing language and insightful writing assistance.
References
1. Adachi K, Murai Y, Teramoto A. Infantile cerebellar pilocytic astrocytoma with autism spectrum disorder. J Nippon Med Sch. 2012. 79: 228-31
2. AlRayahi J, Zapotocky M, Ramaswamy V, Hanagandi P, Branson H, Mubarak W. Pediatric brain tumor genetics: What radiologists need to know. Radiographics. 2018. 38: 2102-22
3. Bonfield CM, Steinbok P. Pediatric cerebellar astrocytoma: A review. Childs Nerv Syst. 2015. 31: 1677-85
4. Chiarelli PA, Chu JK, Krieger MD, editors. Brainstem tumors. Textbook of Pediatric Neurosurgery. Berlin: Springer International Publishing; 2019. p.
5. Cohen AR. Brain tumors in children. N Engl J Med. 2022. 386: 1922-31
6. Cohen KJ, Jabado N, Grill J. Diffuse intrinsic pontine gliomas-current management and new biologic insights. Is there a glimmer of hope? Neuro Oncol 2017. ;. 19: 1025-34
7. Dangouloff-Ros V, Varlet P, Levy R, Beccaria K, Puget S, Dufour C. Imaging features of medulloblastoma: Conventional imaging, diffusion-weighted imaging, perfusion-weighted imaging, and spectroscopy: From general features to subtypes and characteristics. Neurochirurgie. 2021. 67: 6-13
8. Faulkner C, Ellis HP, Shaw A, Penman C, Palmer A, Wragg C. BRAF fusion analysis in pilocytic astrocytomas: KIAA1549-BRAF 15-9 fusions are more frequent in the midline than within the cerebellum. J Neuropathol Exp Neurol. 2015. 74: 867-72
9. Fustinoni O, editors. Semiología del Sistema Nervioso. Argentina: Editorial el Ateneo; 2014. p.
10. Jaremko JL, Jans LB, Coleman LT, Ditchfield MR. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. AJNR Am J Neuroradiol. 2010. 31: 1613-6
11. Komori T. The 2016 WHO classification of tumours of the central nervous system: The major points of revision. Neurol Med Chir (Tokyo). 2017. 57: 301-11
12. Komori T, editors. The 2021 WHO classification of tumors, 5th edition, central nervous system tumors: The 10 basic principles. Brain Tumor Pathol. 2022. 39: 47-50
13. Leland Albright A, editors. Principles and Practice of Pediatric Neurosurgery. New York: Thieme; 2014. p.
14. Levine AB, Wong D, Fatehi M, Yip S. Ependymoma and chordoma. Neurosurgery. 2020. 87: 860-70
15. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
16. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, FigarellaBranger D. The 2021 WHO Classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
17. León MI. Review and update about medulloblastoma in children. Radiología. 2011. 53: 134-45
18. Mosaab A, El-Ayadi M, Khorshed EM, Amer N, Refaat A, El-Beltagy M. Histone H3K27M mutation overrides histological grading in pediatric gliomas. Sci Rep. 2020. 10: 8368
19. Myseros JS, editors. Ependymomas. Textbook of Pediatric Neurosurgery. Berlin: Springer International Publishing; 2018. p.
20. Nesvick CL, Lafay-Cousin L, Raghunathan A, Bouffet E, Huang AA, Daniels DJ. Atypical teratoid rhabdoid tumor: Molecular insights and translation to novel therapeutics. J Neurooncol. 2020. 150: 47-56
21. Osborn AG, Louis DN, Poussaint TY, Linscott LL, Salzman KL. The 2021 World Health Organization classification of tumors of the central nervous system: What neuroradiologists need to know. AJNR Am J Neuroradiol. 2022. 43: 928-37
22. Pollack IF, Agnihotri S, Broniscer A. Childhood brain tumors: Current management, biological insights, and future directions. Neurosurg Pediatr. 2019. 23: 261-73
23. Purves D, editors. Neuroscience. United Kingdom: Oxford University Press, Incorporated.; 2017. p.
24. Ramaswamy V, Remke M, Adamski J, Bartels U, Tabori U, Wang X. Medulloblastoma subgroup-specific outcomes in irradiated children: Who are the true high-risk patients?. Neuro Oncol. 2016. 18: 291-7
25. Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013. 14: 1200-7
26. Raybaud A, Barkovich AJ, editors. Hydrocephalus. Pediatric Neuroimaging. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. p. 808-57
27. Reynolds R, Grant G, editors. General approaches and considerations for pediatric brain tumors. Youmans Neurological Surgery. Philadelphia, PA: Elsevier Inc; 2022. p.
28. Robinson GW, Gajjar A. Genomics paves the way for better infant medulloblastoma therapy. J Clin Oncol. 2020. 38: 2010-3
29. Thompson YY, Ramaswamy V, Diamandis P, Daniels C, Taylor MD. Posterior fossa ependymoma: Current insights. Childs Nerv Syst. 2015. 31: 1699-706
30. Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017. 81: 397-415
31. Wen HT, Rhoton A, Mussi AC, Winn HR, editors. Surgical anatomy of the brain. Youmans Neurological Surgery. Philadelphia, PA: Elsevier Inc; 2016. p.
32. WHO Classification of Tumours Editorial Boa, editors. Central Nervous System Tumours. France: International Agency for Research on Cancer; 2022. 6: