- Department of Neurosurgery, Faculty of Medicine, Cairo University, Cairo, Egypt,
- Department of Neurosurgery, Greifswald Medical School, University of Greifswald, Greifswald, Germany.
Correspondence Address:
Mohammad Elbaroody, Department of Neurosurgery, Cairo University Hospitals, Cairo University, Cairo, Egypt.
DOI:10.25259/SNI_1247_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammed Fathy Adel Ali1, Mohammad Elbaroody1, Mohamed F. M. Alsawy1, Ahmed El Fiki1, Ehab El Refaee1,2, Hesham A. Elshitany1. Postoperative cerebral infarction after evacuation of traumatic epidural hematoma in children younger than two years: Single-center experience. 15-Apr-2022;13:141
How to cite this URL: Mohammed Fathy Adel Ali1, Mohammad Elbaroody1, Mohamed F. M. Alsawy1, Ahmed El Fiki1, Ehab El Refaee1,2, Hesham A. Elshitany1. Postoperative cerebral infarction after evacuation of traumatic epidural hematoma in children younger than two years: Single-center experience. 15-Apr-2022;13:141. Available from: https://surgicalneurologyint.com/surgicalint-articles/11542/
Abstract
Background: Epidural hematoma (EDH) forms about 2–3% of all head injuries in the pediatric population. We evaluated clinical data and risk factors for postoperative infarction in children younger than 2 years presented with traumatic EDH.
Methods: We retrospectively reviewed and analyzed the data of 28 children with traumatic EDH operated in our institute during a period of 26 months (from December 2016 to Febuary 2019).
Results: Nineteen children were boys (68%) and nine were girls (32%), the mean age was 15 months (range from 5 to 24 months). Postoperative cerebral infarction was detected in seven cases (25%). Factors could be linked to postoperative cerebral: preoperative pediatric Glasgow Coma Scale (P = 0.036), neurological deficit on admission (P = 0.023), size of hematoma (P P = 0.004), midline shift (MLS) (P = 0.001), and basal cistern compression (P = 0.004).
Conclusion: Traumatic EDH in young children represents a neurosurgical challenge that needs rapid surgical intervention for the best surgical outcome. Delay in the time of surgery for more than 6 h, large hematoma volume >100 ml3, MLS >10 mm, and basal cisterns compression will push the intracranial pressure to the point of decompensation and the resultant ischemic sequel occurs.
Keywords: Epidural hematoma, Extradural hematoma, Head injury, Infants, Infarction
INTRODUCTION
Epidural hematoma (EDH) is a common type of traumatic brain injury in adults but it represents a rare clinical and pathological entity in children.[
MATERIALS AND METHODS
Patients
We retrospectively reviewed and analyzed the collected data of 28 children under the age of 2 years with traumatic acute EDH who were surgically treated in our institute during a period of 26 months (from December 2016 to February 2019). We specified inclusion and exclusion criteria for patients to be encountered in this study, the inclusion criteria were: (1) pediatric age group ≤2 years, (2) computed tomography (CT) brain without contrast showing evidence of traumatic EDH, and (3) EDH managed with craniotomy in our department. Exclusion criteria were: (1) children older than 2 years, (2) coexistent subdural hematoma, brain contusions, or lacerations, (3) patients operated outside our institute.
Radiological criteria
CT brain was done for all children before admission.[
Clinical indices
We evaluated 12 clinical indices: gender, age, mode of trauma, clinical presentation, preoperative pediatric Glasgow Coma Scale (GCS), the neurological deficit on admission, preoperative hemoglobin level, location of EDH, the volume of hematoma, basal cisterns morphology, midline shift, and timing from trauma to surgery.
Treatment
Indications for emergency surgery were based on the following: (1) an EDH >30 ml3 with 15-mm thickness and more than a 5-mm MLS and (2) hematoma with a maximum diameter of more than 18 mm and MLS more than 5 mm.[
Postoperative CT brain was done for patients on the 1st and 7th day and postoperative cerebral infarction was identified as a low-density area along with distribution of posterior cerebral artery (PCA) as a sequela for uncal herniation.
The mean time for follow-up in our series was 5 months (from 4 to 6 months), where we followed the clinical status of the patients who developed infarction and started on physiotherapy.
Ethics approval and consent to participate
Patient confidentiality was kept following the Helsinki Declaration. Due to its retrospective nature, ethical board approval was not obtained, especially that there were no specific interventions for the patients enlisted in our study.
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient’s guardian has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patient’s guardian understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Statistical analysis
Microsoft Excel® 2013 (Microsoft® Corporation, Redmond, WA) was used for data entry and the Statistical Package for the Social Science (SPSS®, Armonk, New York: International Business Machines Corporation) was used for data analysis. All collected data were revised for competencies and logical consistency. Simple descriptive statistics (arithmetic mean and standard deviation) are used for the summary of normal quantitative data and frequencies used for qualitative data. The bivariate relationship was displayed in cross-tabulations and a comparison of proportions was performed using the Chi-square and Fisher’s exact tests where appropriate. An independent t-test was used to compare normally distributed quantitative data. Those factors that were significantly associated with infarction in bivariate analysis (P < 0.05) were included in multivariate logistic regression models. The level of significance was set at a probability P < 0.05.
Case presentation
Case 1
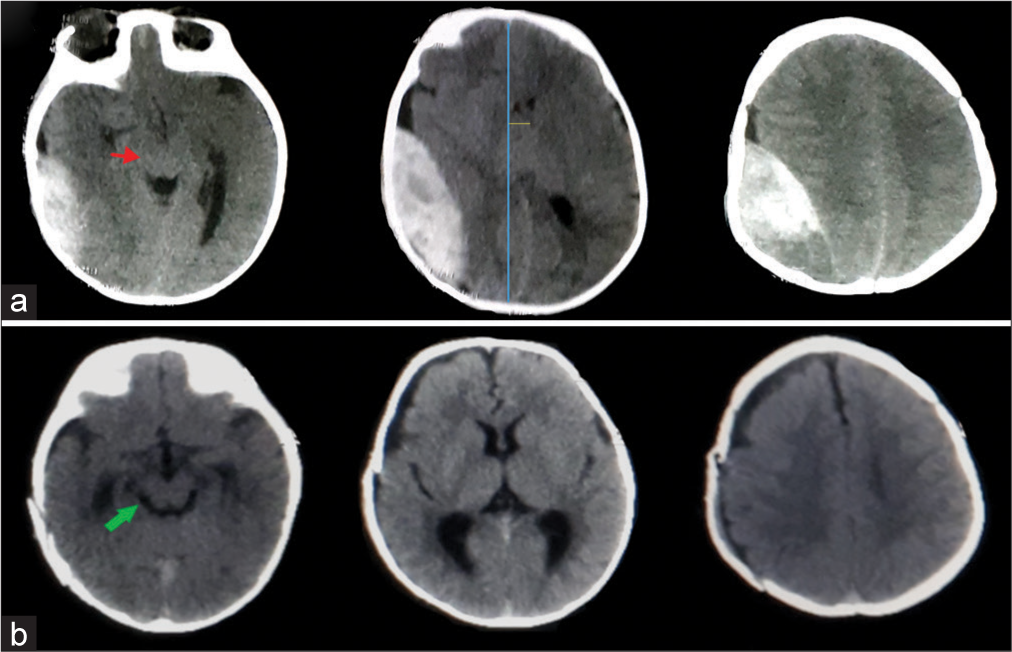
A 6-month-old male child falls from a height on his head presented with a decrease in consciousness level, CT brain was done and showed a large right temporoparietal occipital EDH [
Figure 1:
CT brain, axial cuts without contrast (a) right temporoparietal occipital epidural hematoma, red arrow; brainstem and cisternal compression, and blue and yellow lines highlight the midline shift. (b) Postoperative CT after hematoma evacuation, green arrow points to relief of brainstem after surgery.
Case 2
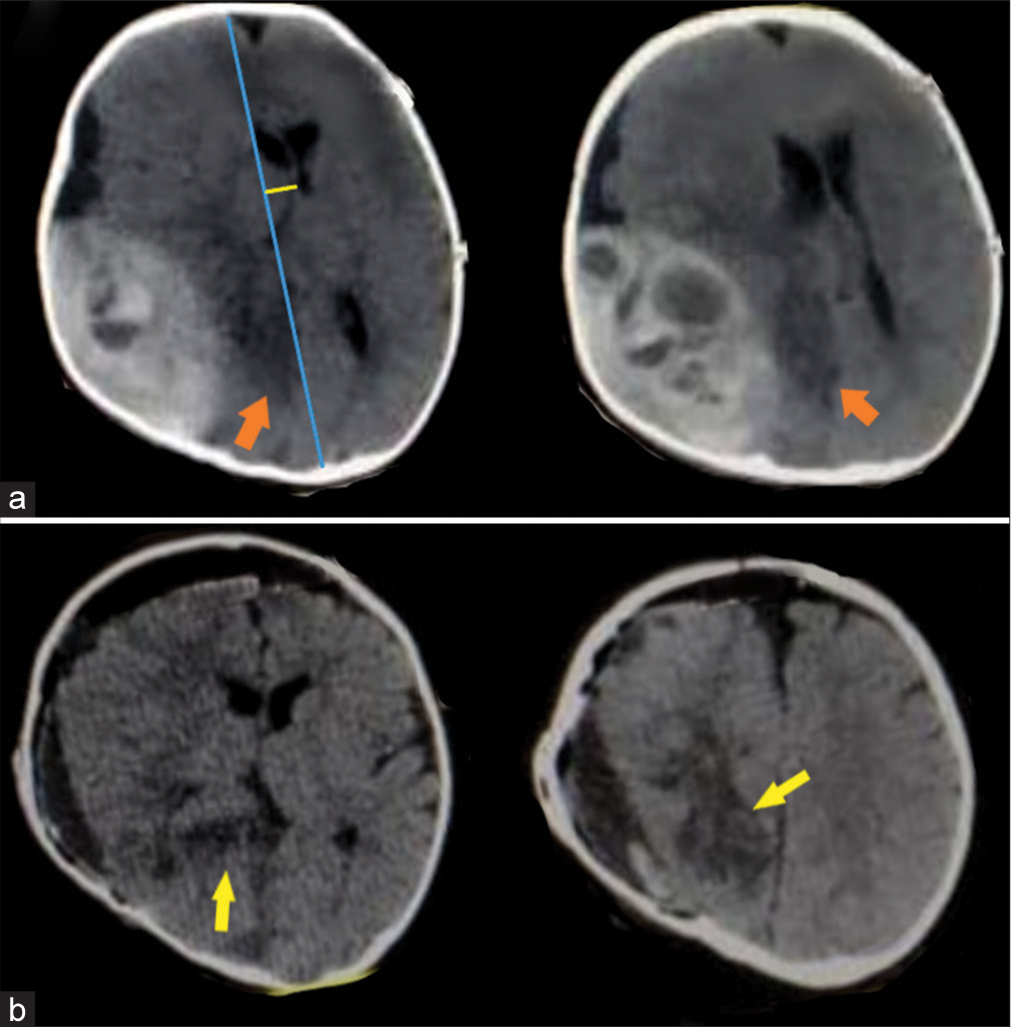
An 8-month-old male child falls from a height on his head presented with a decrease in consciousness level, CT brain was done and showed a large right temporoparietal occipital EDH with active bleeding [
Figure 2:
CT brain, axial cuts without contrast, (a) right temporoparietal hyperacute epidural hematoma with active bleeding, orange arrows hypodense areas in distribution of posterior cerebral artery from severe compression due to herniation of temporal lobe, and blue and yellow lines highlight the midline shift. (b) Postoperative CT, yellow arrows point to the infarction in the form of hypodense areas.
Case 3
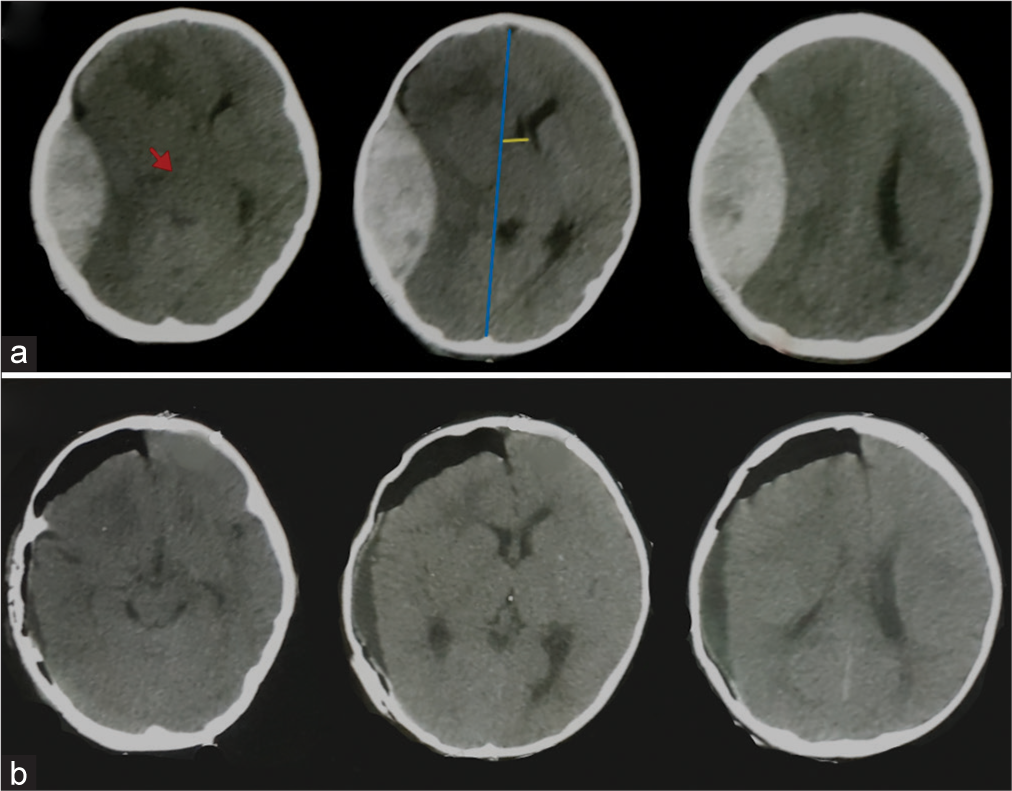
A 9-month-old female child falls on the ground presented with repeated vomiting, CT brain done and showed a large right temporoparietal EDH [
RESULTS
Patient’s characteristics
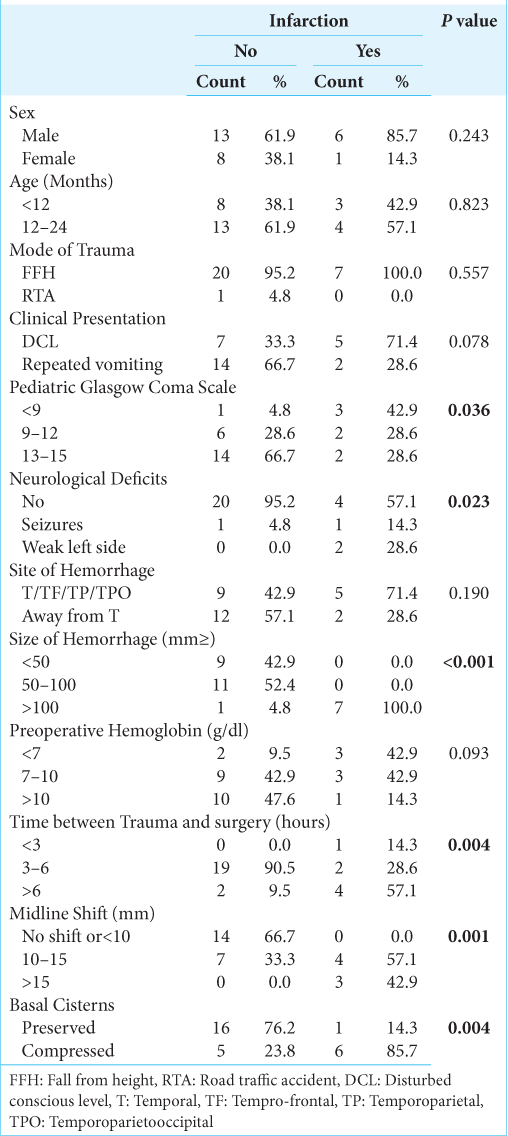
Nineteen children were boys (68%) and nine were girls (32%), the mean age was 15 months (range from 5 to 24 months), the mean pediatric GCS on admission was 12 (range from 5 to 15), the most common mode of trauma was domestic fall from a height which happened in 27 cases, and only one child came in a road traffic accident. Repeated vomiting was the main symptom in 16 children (57%) while loss of consciousness was the presentation in the other 12 cases (43%). The time interval between trauma and surgery varied from 3 to 16 h (mean 5.4 h).
Gender, age, mode of trauma, and clinical presentation had no statistically significant relation to postoperative infarction (P = 0.243, 0.823, 0.557, and 0.078, respectively). The mean preoperative hemoglobin was 8.89 g/dl (average from 3.9 to 11.8 g/dl) (P = 0.093).
The pediatric GCS on admission is strongly correlated with postoperative infarction (P = 0.036). On discharge, the GCS turned out to be independent of the initial GCS after trauma whenever the initial GCS was 6 or more, so that 27 cases recovered completely, and one case discharged in the vegetative state (P =0.006).
19/21 of patients who did not develop infarction operated on within 3–6 h, while 4/7 of patients who developed infarction were operated on after 6 h from the time of trauma (P = 0.004).
At the time of presentation, 20/21 (92.2%) of patients who did not develop infarction and 4/7 (57.1%) of patients who developed infarction did not show any neurological deficit (P = 0.023).
Radiological data
The hematoma was related to the temporal lobe (temporal, temporoparietal, temporoparietal-occipital, and frontotemporal) in 14 cases (50%) and it was away from the temporal lobe in the other 14 cases. The mean volume of hematoma was 72.75 ml3 (range from 30 to 140 ml3), there were eight cases had a preoperative hematoma volume >100 ml3, seven out of eight developed postoperative infarction (P < 0.001).
Postoperatively, an ipsilateral cerebral infarction was identified in the form of hypodense areas along the distribution of PCA in seven children (25%).
The MLS was one of the important parameters that also indicated the occurrence of ipsilateral infarction, it was more than 10 mm in 14 cases (50%) and <10 mm in the other 14 children and similarly, all cases that developed infarction had MLS more than 10 mm (P = 0.001). The basal cisterns were compressed in 6/7 (85.7%) of patients who developed infarction (P = 0.004).
[
DISCUSSION
EDH in children and especially in infants represents a rare and potentially life-threatening complication resulting from head injuries.[
In our study, the most common mode of trauma in children was domestic fall from height in 27 cases (96.4%) which was concomitant with the same mode of trauma in many published series,[
It was reported that temporoparietal and temporal regions are the most common locations of EDH in this age group.[
The importance of this temporal location, in particular, stems from its proximity to perimesencephalic cisterns and PCA branches, so that a hematoma in this site may have more possibilities to yield an infarction by distorting the cisterns with resultant compression of posterior circulation arteries against the tentorium by the herniating brain especially in children whose blood vessels are more elastic and more liable to be kinked.[
The size of hematoma in relation to total blood volume in infants is the reason behind the apparent anemia in infants, it is estimated that children more than 3 months have 70 ml/kg as a total volume of blood.[
Pasaoglu et al. reported that pallor and anemia occurred in nine out of ten infants in traumatic pediatric EDH series,[
The preoperative low hemoglobin was not linked to infarction development (P = 0.093), it was <7 g/dl in 3/7(42.7%) of children with infarction, and 1/7 (14.3%) of cases with infarction had preoperative hemoglobin >10 g/dl. The mean preoperative hemoglobin was 8.89 gm/dl (average from 3.9 to 11.8 g/dl) (P = 0.093), this means that even with children presented with low hemoglobin <7 g/dl, the on-table intraoperative correction by anesthesia early surgery through blood transfusion made those cases salvageable from infarction. [
MLS larger than 10 mm is usually associated with unfavorable outcomes due to cisternal compression with mechanical dragging and kinking of blood vessels,[
There was a relationship between the timing of surgery to head trauma and development of infarction (P = 0.004), about 90.5% of patients who did not develop infarction were operated on between 3 and 6 h from the time of trauma. Delay in the timing of surgery carries a great risk and to increase intracranial pressure (ICP), more than 50% of patients who developed infarction were operated on after 6 h from the time of trauma.
According to the ICP-volume curve, despite little addition of volume, the ICP remains the same until the point of decompensation where ICP rises incrementally.[
In our series, we tried to elucidate factors that could be linked to infarction from our tertiary-based practice. The time needed to reach the hospital is out of our hands especially in developing countries, but rapid correction of low hemoglobin and rapid surgical evacuation is the best to be done to salvage the brain. Up to 6 h could be the time limit after which infarction is inevitable.
Limitations
Being retrospective rather than a prospective study, the relatively small sample size is the main limitation of the study; however, the sample size was comparable to other pediatric EDH series[
CONCLUSION
Pediatric EDH is a surgical emergency that needs rapid intervention to achieve the best outcome. Parameter to predict the occurrence of ipsilateral infarction is GCS on admission, the presence of neurological deficit; the size of the hematoma, midline shift; compression of basal cisterns, and timing of surgery. Intraoperative rapid correction of low preoperative hemoglobin could salvage the brain from ischemic sequelae.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abraham RB, Lahat E, Sheinman G, Feldman Z, Barzilai A, Harel R. Metabolic and clinical markers of prognosis in the era of CT imaging in children with acute epidural hematomas. Pediatr Neurosurg. 2000. 33: 70-5
2. Ammirati M, Tomita T. Epidural hematomas in infancy and childhood. Riv Neurosci Pediatr. 1985. 1: 123-8
3. Andrews PJD, Citerio G. Intracranial pressure. Intensive Care Med. 2004. 30: 1730-3
4. Bejjani GK, Donahue DJ, Rusin J, Broemeling LD. Radiological and clinical criteria for the management of epidural hematomas in children. Pediatr Neurosurg. 1996. 25: 302-8
5. Beni-Adani L, Flores I, Spektor S, Umansky F, Constantini S. Epidural hematoma in infants: A different entity?. J Trauma Acute Care Surg. 1999. 46: 306
6. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of traumatic brain injury. Neurosurgery. 2006. 58: 16-24
7. Chen TY, Wong CW, Chang CN, Lui TN, Cheng WC, Tsai MD. The expectant treatment of “asymptomatic” supratentorial epidural hematomas. Neurosurgery. 1993. 32: 176-9
8. Choux M, Grisoli F, Peragut JC. Extradural hematomas in children. Childs Brain. 1975. 1: 337-47
9. Ciurea AV, Kapsalaki EZ, Coman TC, Roberts JL, Robinson JS, Tascu A. Supratentorial epidural hematoma of traumatic etiology in infants. Childs Nerv Syst. 2007. 23: 335-41
10. Colantonio A, Croxford R, Farooq S, Laporte A, Coyte PC. Trends in hospitalization associated with traumatic brain injury in a publicly insured population, 1992-2002. J Trauma Acute Care Surg. 2009. 66: 179-83
11. Dehmer JJ, Adamson WT. Massive transfusion and blood product use in the pediatric trauma patient. Semin Pediatr Surg. 2010. 19: 286-91
12. Dhellemmes P, Lejeune JP, Christiaens JL, Combelles G. Traumatic extradural hematomas in infancy and childhood: Experience with 144 cases. J Neurosurg. 1985. 62: 861-4
13. Erşahin Y, Mutluer S, Güzelbag E. Extradural hematoma: Analysis of 146 cases. Childs Nerv Syst. 1993. 9: 96-9
14. Gallagher JP, Browder EJ. Extradural hematoma: Experience with 167 patients. J Neurosurg. 1968. 29: 1-12
15. Holmes JF, Palchak MJ, MacFarlane T, Kuppermann N. Performance of the pediatric glasgow coma scale in children with blunt head trauma. Acad Emerg Med. 2005. 12: 814-9
16. Leggate JR, Lopez-Ramos N, Genitori L, Lena G, Choux M. Extradural haematoma in infants. Br J Neurosurg. 1989. 3: 533-9
17. Maggi G, Aliberti F, Petrone G, Ruggiero C. Extradural hematomas in children. J Neurosurg Sci. 1998. 42: 95
18. Mazza C, Pasqualin A, Feriotti G, Da Pian R. Traumatic extradural haematomas in children: Experience with 62 cases. Acta Neurochir. 1982. 65: 67-80
19. Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H. Surgical outcome following a decompressive craniectomy for acute epidural hematoma patients presenting with associated massive brain swelling. Acta Neurochir Suppl. 2010. 106: 261-4
20. Paşaoglu A, Orhon C, Koç K, Selçuklu A, Akdemir H, Uzunoglu H. Traumatic extradural haematomas in pediatric age group. Acta Neurochir. 1990. 106: 136-9
21. Pillay R, Peter JC. Extradural haematomas in children. S Afr Med J. 1995. 85: 672-4
22. Rashmi UK, Thomas B, Joseph PB, William GB, Laura RS, Mario Z. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996. 27: 1304-5
23. Rocchi G, Caroli E, Raco A, Salvati M, Delfini R. Traumatic epidural hematoma in children. J Child Neurol. 2005. 20: 569-71
24. Ulrich PT, Fuessler H, Januschek E. Acute epidural hematoma with infarction of the right hemisphere in a 5-month-old child: Case report with a long-term follow-up and a review of the literature. J Child Neurol. 2008. 23: 1066-9
25. Wang W, Hu L, Lin H, Li J, Luo F, Huang W. Risk factors for post-traumatic massive cerebral infarction secondary to space-occupying epidural hematoma. J Neurotrauma. 2014. 31: 1444-50
26. Xiaoyu W, Guoping L. Surgical treatment of supraand infratentorial epidural hematoma. Turk Neurosurg. 2013. 23: 299-303
27. Zhu Y, Qi S, Wu H, Xiang Z. Study on the improvement of coniglobus formula based on subdural hematoma volume estimates. Neurosurg Q. 2015. 25: 275-9