- Department of Neurosurgery, Medical Research Institute KITANO HOSPITAL, Osaka, Japan
- Department of Neurosurgery, Hikone Chuo Hospital, Hikone, Japan.
Correspondence Address:
Hirokuni Hashikata, Department of Neurosurgery, Medical Research Institute KITANO HOSPITAL, Osaka, Japan.
DOI:10.25259/SNI_397_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hirokuni Hashikata1, Noriyoshi Takebe1, Wataru Yoshizaki1, Yoshinori Maki2. Postoperative vasospasm and cerebral infarction in a patient with large pituitary adenoma and cerebral superficial siderosis. 21-Jul-2023;14:256
How to cite this URL: Hirokuni Hashikata1, Noriyoshi Takebe1, Wataru Yoshizaki1, Yoshinori Maki2. Postoperative vasospasm and cerebral infarction in a patient with large pituitary adenoma and cerebral superficial siderosis. 21-Jul-2023;14:256. Available from: https://surgicalneurologyint.com/surgicalint-articles/12449/
Abstract
Background: Cerebral vasospasm and infarction are rare complications of transsphenoidal surgery for pituitary adenoma. Cerebral superficial siderosis may result from subarachnoid hemorrhage from a pituitary adenoma. The constellation of cerebral superficial siderosis, cerebral vasospasm, and pituitary adenoma is rare. We describe an extremely rare clinical constellation of immediately postoperative cerebral vasospasm and consequent cerebral infarction in a case with a large pituitary adenoma and cerebral superficial siderosis.
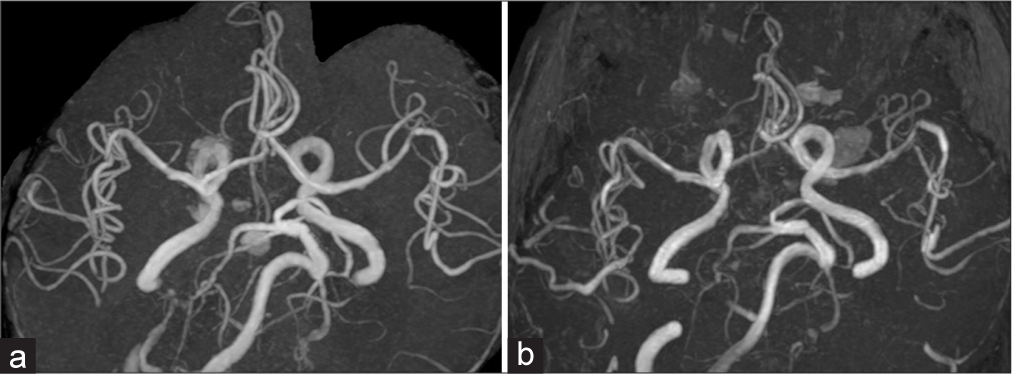
Case Description: A 70-year-old man presented with a pituitary adenoma causing a worsening headache. Preoperative magnetic resonance (MR) images revealed cerebral superficial siderosis, suggesting subarachnoid hemorrhage from pituitary apoplexy. MR angiography (MRA) showed no vasospasm. During the transsphenoidal surgery, an intratumoral hematoma was found. The arachnoid membrane was partially torn and intratumoral hematoma entered the subarachnoid space. Intraoperatively, the intracranial vessels remained intact. The suprasellar tumor was almost entirely resected; however, the patient remained comatose postoperatively. Computed tomography revealed ischemic lesions in the bilateral insular and frontotemporal cortex. MRA revealed cerebral vasospasm in the bilateral middle cerebral arteries. The patient was treated with levetiracetam for nonconvulsive status epilepticus and underwent a lumbar peritoneal shunt surgery for secondary hydrocephalus. However, the patient remained listless.
Conclusion: Postoperative cerebral vasospasm and infarction are severe but rare complications for a pituitary adenoma after transsphenoidal surgery. Preoperative and intraoperative subarachnoid hemorrhage might have been a risk factor in our case. Similar cases should be warranted to analyze whether cerebral superficial siderosis may also indicate the risk of severe postoperative vasospasm immediately after transsphenoidal surgery for pituitary adenoma.
Keywords: Cerebral superficial siderosis, Complication, Ischemic, Pituitary adenoma, Transnasal endoscopic transsphenoidal surgery, Vasospasm
INTRODUCTION
The complication rates related to microscopic and endoscopic transsphenoidal surgery for pituitary adenomas vary from 8.4% to 10.2%. Common postoperative complications include cerebrospinal fluid leakage, cerebrovascular injury, cranial nerve palsy, intracranial hematoma, meningitis, pituitary insufficiency, and visual impairment.[
Superficial cerebral siderosis can manifest as hearing impairment, gait ataxia, and cognitive impairment.[
CASE PRESENTATION
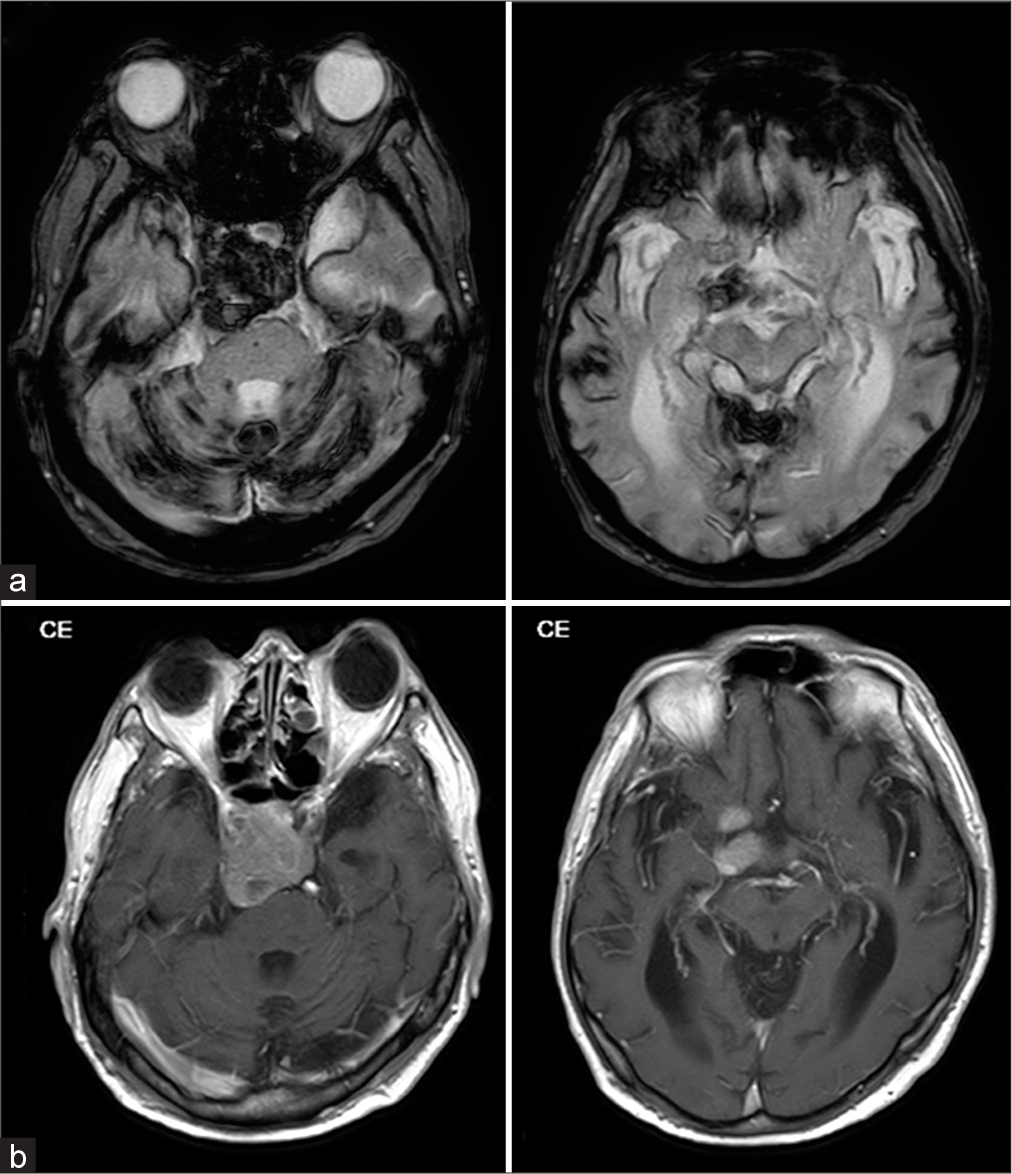
A 70-year-old man presented with a worsening headache. The patient was suffering from aggravated dementia, diplopia, right dysplasia, and gait disturbance for 6 months. On hospital admission, magnetic resonance imaging (MRI) revealed a tumor in the sella turcica; however, neurological and ophthalmological examinations did not reveal apparent visual loss. The diplopia was confirmed through vertical and horizontal eye movements. The revised Hasegawa dementia scale (HDS-R) and functional independence measure scores were 22 and 109, respectively. Laboratory tests revealed that pituitary hormone was normal. Further MRI revealed homogeneous tumor enhancement, intratumoral hemorrhage, and the tumor extended into the clivus. The cerebrum and cerebellum were atrophic and superficial cerebral siderosis was confirmed in both hemispheres [
Figure 1:
Preoperative magnetic resonance images. (a) Atrophic change observed in the cerebrum and cerebellum. Cerebral superficial siderosis in the bilateral frontal lobes and cerebellar hemispheres, and hemorrhagic change in the tumor were also identified. (b) The tumor in the sella turcica extends close to the brain stem (a: T2 star-weighted images, and b: T1-weighted gadolinium-enhanced images).
Transnasal endoscopic transsphenoidal surgery
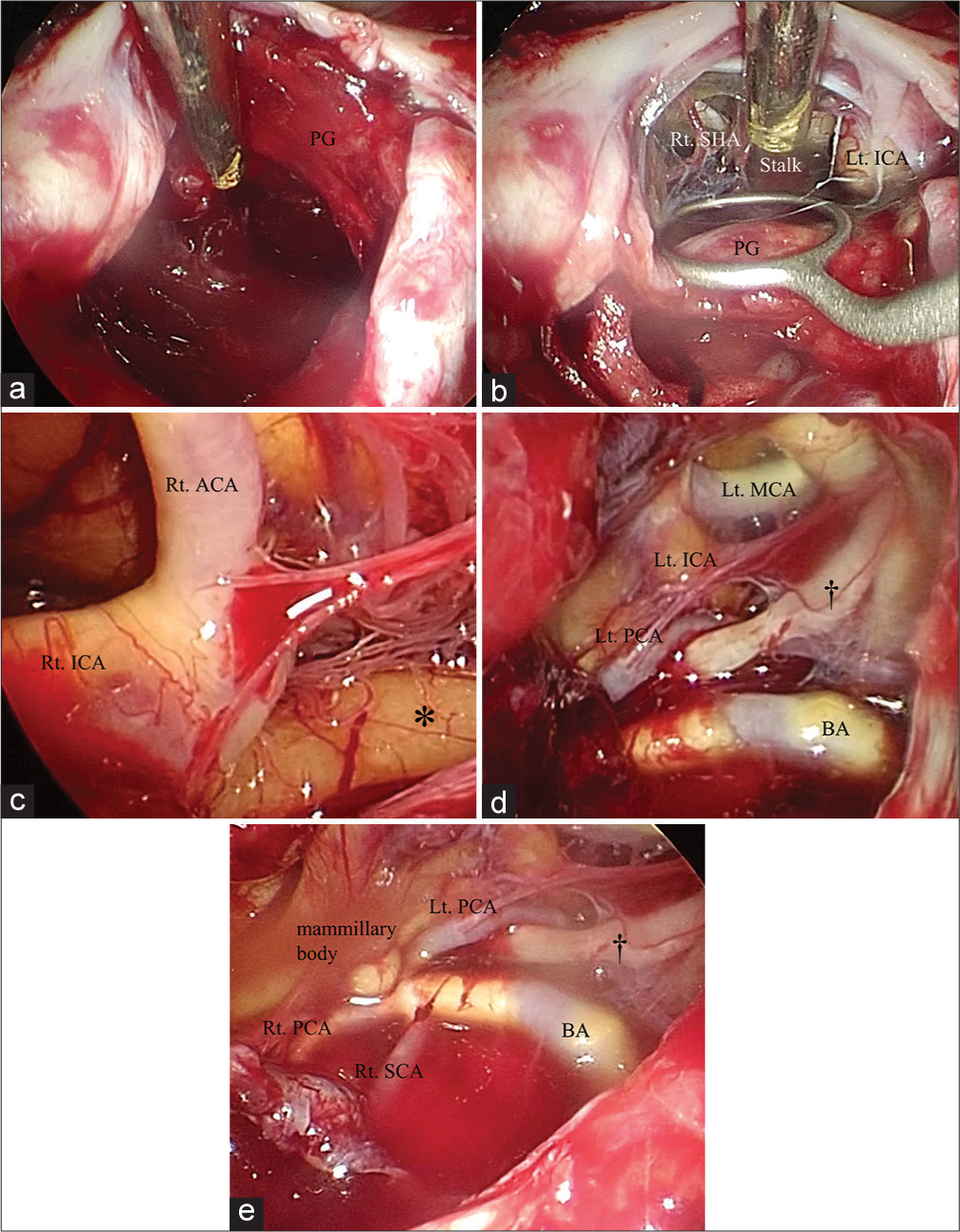
The patient underwent extended transnasal endoscopic transsphenoidal surgery in the supine position under general anesthesia. After preparing a pedicled nasal septal flap and opening the sphenoid sinus, we found a thin wall in the sella turcica and clivus. A dural incision was made and the tumor containing the acute and chronic hematomas was exposed [
Figure 2:
Intraoperative findings under neuroendoscope (a-c, d: under a neuroendoscope angled at 30°, e: under a neuroendoscope angled at 0°). (a) Intratumoral hematoma is observed intraoperatively. The pituitary gland is compressed laterally by the tumor. (b) The pituitary gland and its stalk are preserved intraoperatively. (c-e) After the removal of the tumor in the sella turcica and posterior cranial fossa, the vascular and nerve structures are observed. Intraoperatively, they were preserved. Hemosiderin (asterisk) attaches to the brain surface. The left oculomotor nerve (dagger) is also identified. ACA: Anterior cerebral artery, BA: Basilar artery, ICA: Internal carotid artery, Lt: Left, MCA: Middle cerebral artery, PCA: Posterior cerebral artery, PG: Pituitary gland, Rt: Right, SCA: Superior cerebellar artery, SHA: Superior hypophyseal artery.
Postoperative course
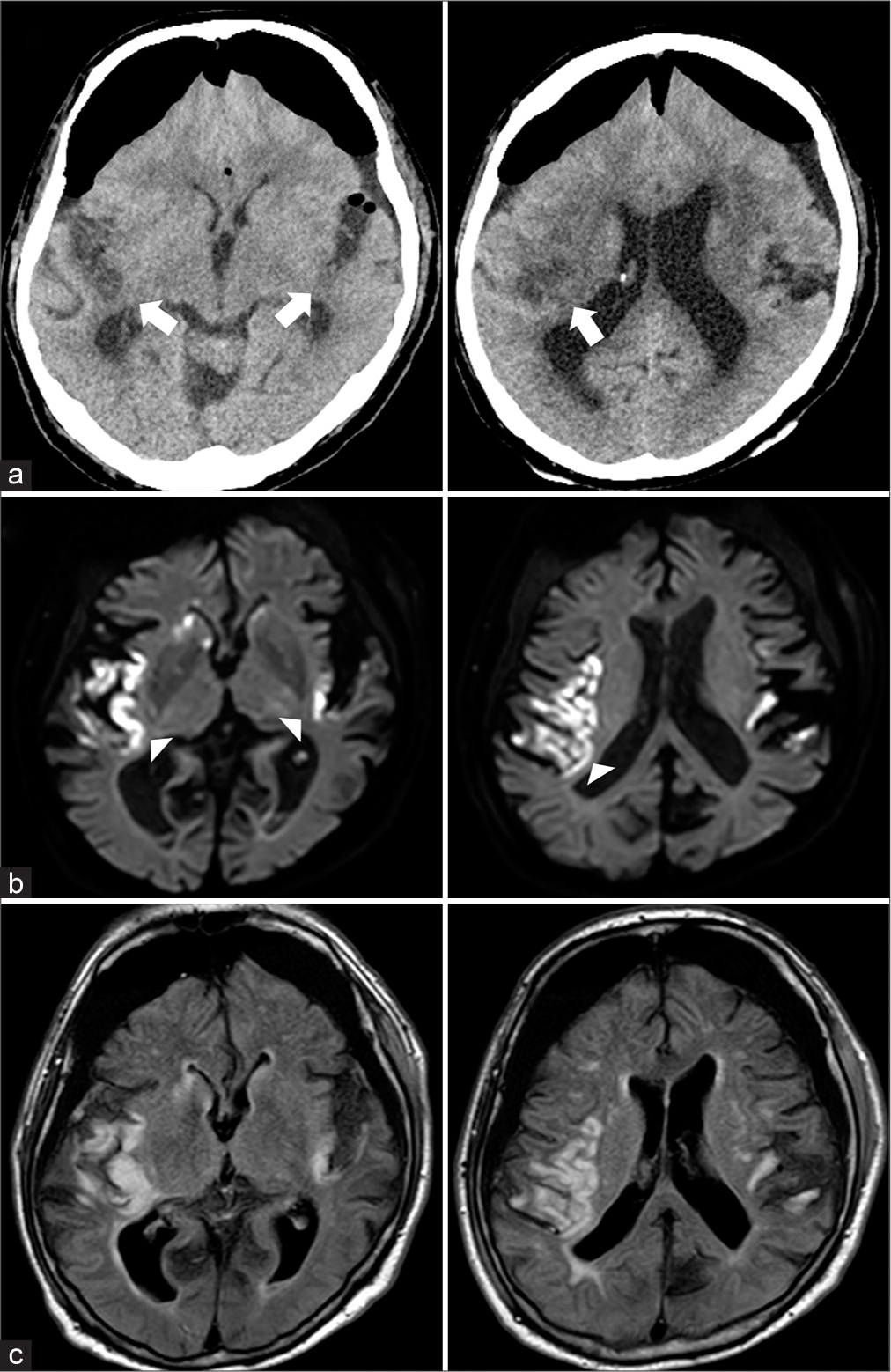
After surgery, the patient remained intubated and unconscious. Postoperative CT imaging on postoperative day 1 showed pneumocephalus and low-density areas in the bilateral insular and frontotemporal cortices, with no intracranial hemorrhage [
Figure 3:
Postoperative radiological images. (a) Computed tomography images on a postoperative day 1 show large pneumocephalus and diffuse ischemic lesions in the bilateral insulae and cerebral cortices (white arrows), (b and c) Acute ischemic lesions in the insula, temporal, and frontal cortices are disclosed bilaterally (white arrow heads). Subarachnoid hemorrhage is not observed. (b) diffusion-weighted images, and (c) fluid-attenuated inversion recovery images.
DISCUSSION
Here, we describe a case of postoperative cerebral vasospasm and infarction following transsphenoidal surgery for a nonfunctioning pituitary adenoma, concurrently diagnosed with preoperative cerebral superficial siderosis. Postoperatively, the patient remained unconscious, possibly due to cerebral infarctions in the left thalamus and bilateral cerebral hemispheres, including the insular cortices. In addition, consecutive NCSE appeared to have persisted as a state of impaired consciousness in the patient.
Our case is unique because asymptomatic superficial cerebral siderosis was preoperatively identified. Superficial cerebral siderosis can manifest neurological deficits,[
As for the pathophysiology of postoperative cerebral vasospasm in our case, several mechanisms seem involved. Considering the patient’s age of 70, surgical manipulation stress, prolonged intraoperative hypotension, and underlying atherosclerosis could have also facilitated the occurrence of postoperative cerebral vasospasm. In our case, the arachnoid membrane was partially torn, when the tumor was surgically detached from the surrounding structures. Although no apparent subarachnoid hemorrhage was observed on postoperative radiological images, minor intraoperative hemorrhagic inflow into the subarachnoid space could have been a cause of the symptomatic cerebral vasospasm in our case.[
NCSE likely results in prolonged unconsciousness. Since cerebral superficial siderosis and pneumocephalus are considered risk factors for NCSE, the concurrent pathology in our case may have resulted in an unfavorable outcome.[
To the best of our knowledge, this is the first reported case of cerebral vasospasm and infarction after transsphenoidal surgery for a pituitary tumor presenting concurrently with superficial cerebral siderosis. However, the correlation between these pathologies has not yet been elucidated. Therefore, attention should be paid to the possible risk of postoperative cerebral spastic and ischemic complications after transsphenoidal surgery for pituitary tumors with concurrent cerebral superficial siderosis. Further studies are required to confirm this hypothesis.
CONCLUSION
We describe a rare case of postoperative cerebral vasospasm resulting in ischemic complications after transsphenoidal surgery for a nonfunctioning pituitary adenoma concurrently diagnosed with preoperative cerebral superficial siderosis. Similar reports should be addressed to clarify the perioperative correlation between preoperative superficial cerebral siderosis and postoperative cerebral vasospasm after transsphenoidal surgery for pituitary adenoma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alotaibi NM, Lanzino G. Cerebral vasospasm following tumor resection. J Neurointerv Surg. 2013. 5: 413-8
2. Budnick HC, Tomlinson S, Savage J, Cohen-Gadol A. Symptomatic cerebral vasospasm after transsphenoidal tumor resection: Two case reports and systematic literature review. Cureus. 2020. 12: e8171
3. Burton TM, Bushnell CD. Reversible cerebral vasoconstriction syndrome. Stroke. 2019. 50: 2253-8
4. Charidimou A, Boulouis G, Pasi M, Gokcal E, Martinez-Ramirez S, Warren AB. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022. 21: 714-25
5. Eseonu CI, Refaey K, Geocadin RG, Quinones-Hinojosa A. Postoperative cerebral vasospasm following transsphenoidal pituitary adenoma surgery. World Neurosurg. 2016. 92: 7-14
6. Harskamp NJ, Rudge P, Cipolotti L. Cognitive and social impairments in patients with superficial siderosis. Brain. 2005. 128: 1082-92
7. Kagami Y, Saito R, Kawataki T, Ogiwara M, Hanihara M, Kazama H. Nonconvulsive status epilepticus due to pneumocephalus after suprasellar arachnoid cyst fenestration with transsphenoidal surgery: Illustrative case. J Neurosurg Case Lessons. 2022. 4: CASE22167
8. Kim EH, Oh MC, Kim SH. Angiographically documented cerebral vasospasm following transsphenoidal surgery for pituitary tumors. Pituitary. 2013. 16: 260-9
9. Kumar N. Superficial siderosis: A clinical review. Ann Neurol. 2021. 89: 1068-79
10. Mapaga JN, Martinez M. Hemosiderosis of the central nervous system and its association with a pituitary macroadenoma: A case report. Pan Afr Med J. 2020. 37: 288
11. Osterhage K, Czorlich P, Burkhardt TR, Rotermund R, Grzyska U, Flitsch J. Symptomatic vasospasms as a life-threatening complication after transsphenoidal surgery. World Neurosurg. 2018. 110: 180-8
12. Popugaev KA, Savin IA, Lubnin AU, Goriachev AS, Kadashev BA, Kalinin PL. Unusual cause of cerebral vasospasm after pituitary surgery. Neurol Sci. 2011. 32: 673-80
13. Puri AS, Zada G, Zarzour H, Laws E, Frerichs K. Cerebral vasospasm after transsphenoidal resection of pituitary macroadenomas: Report of 3 cases and review of the literature. Neurosurgery. 2012. 71: 173-80 discussion 180-1
14. Shida N, Nakasato N, Mizoi K, Kanaki M, Yoshimoto T. Symptomatic vessel narrowing caused by spontaneous rupture of craniopharyngioma cyst--case report. Neurol Med Chir (Tokyo). 1998. 38: 666-8
15. Steinberg J, Cohen JE, Gomori JM, Fraifeld S, Moscovici S, Rosenthal G. Superficial siderosis of the central nervous system due to chronic hemorrhage from a giant invasive prolactinoma. J Clin Neurosci. 2013. 20: 1032-4
16. Sydlowski SA, Levy M, Hanks WD, Clark MD, Ackley RS. Auditory profile in superficial siderosis of the central nervous system: A prospective study. Otol Neurotol. 2013. 34: 611-9
17. Tabaee Damavandi P, Storti B, Fabin N, Bianchi E, Ferrarese C, DiFrancesco JC. Epilepsy in cerebral amyloid angiopathy: An observational retrospective study of a large population. Epilepsia. 2023. 64: 500-10