- Department of Neurosurgery, Maastricht University Medical Center, Maastricht, Limburg, Netherlands,
- Department of Neurosurgery, Helsinki University Hospital, University of Helsinki, Helsinki, Finland.
Correspondence Address:
Roel Hubert Louis Haeren, Department of Neurosurgery, Maastricht University Medical Center, Maastricht, Limburg, Netherlands.
DOI:10.25259/SNI_193_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Roel Hubert Louis Haeren1, Behnam Rezai Jahromi2, Mika Niemela2. Posttraumatic subarachnoid hemorrhage related to concomitant carotid artery dissection and ruptured basilar trunk aneurysm: A case report and literature review. 12-Jul-2021;12:344
How to cite this URL: Roel Hubert Louis Haeren1, Behnam Rezai Jahromi2, Mika Niemela2. Posttraumatic subarachnoid hemorrhage related to concomitant carotid artery dissection and ruptured basilar trunk aneurysm: A case report and literature review. 12-Jul-2021;12:344. Available from: https://surgicalneurologyint.com/surgicalint-articles/10964/
Abstract
Background: Carotid artery dissections (CADs) are a relatively rare disorder, whereas intracranial aneurysms (IAs) form a common cerebrovascular pathology. Since both vascular entities share similar risk factors and associations with connective tissue and vascular disorders, a common pathogenesis has been suggested. Here, we present a case of the concomitant occurrence of a CAD and a ruptured basilar trunk aneurysm (BTA). In the discussion, we elaborate on both vascular entities and have reviewed the literature on their concomitant incidence and potential shared pathogenesis.
Case Description: We present a case of a 40-year-old female patient who was admitted to our hospital because of subarachnoid hemorrhage following a minor head trauma. Imaging revealed a BTA and unilateral extracranial dissection of the internal carotid artery. Despite coiling of the aneurysm, stenting of the dissection, and antithrombotic therapy, the patient died due to extensive cerebral ischemia sequelae.
Conclusion: CAD and BTAs have both been associated with a vascular vulnerability but their concomitant occurrence has not been described previously. The previous studies have suggested an increased incidence of IAs in patients with a CAD and vice versa. However, the number of studies and reports on this mutual increased incidence is limited. Therefore, a shared pathogenesis seems rather speculative. In our case, we suggest that a posttraumatic CAD-induced hemodynamic alterations resulting in rupture of the saccular BTA.
Keywords: Basilar trunk aneurysm, Carotid artery dissection, Intracranial aneurysm, Subarachnoid hemorrhage, Trauma
INTRODUCTION
Carotid artery dissections (CADs) are relatively rare and a preceding trauma is reported in a minority of patients.[
CASE DESCRIPTION
A 40-year-old female with a medical history of HSP and smoking was admitted to our emergency department. The patient was tackled during ice hockey practice and fell on the ice. Thereafter, she skated to the bench where she suddenly lost consciousness. Resuscitation was started immediately with return of spontaneous circulation after 12 min. On arrival of the ambulance, the patient was found unconscious with both pupils dilated and was intubated immediately. On admission, her Glasgow Coma Scale (GCS) was 3 (E1M1V1) with anisocoria. Subsequent computed tomography (CT) scan of the head and neck showed diffuse SAH [
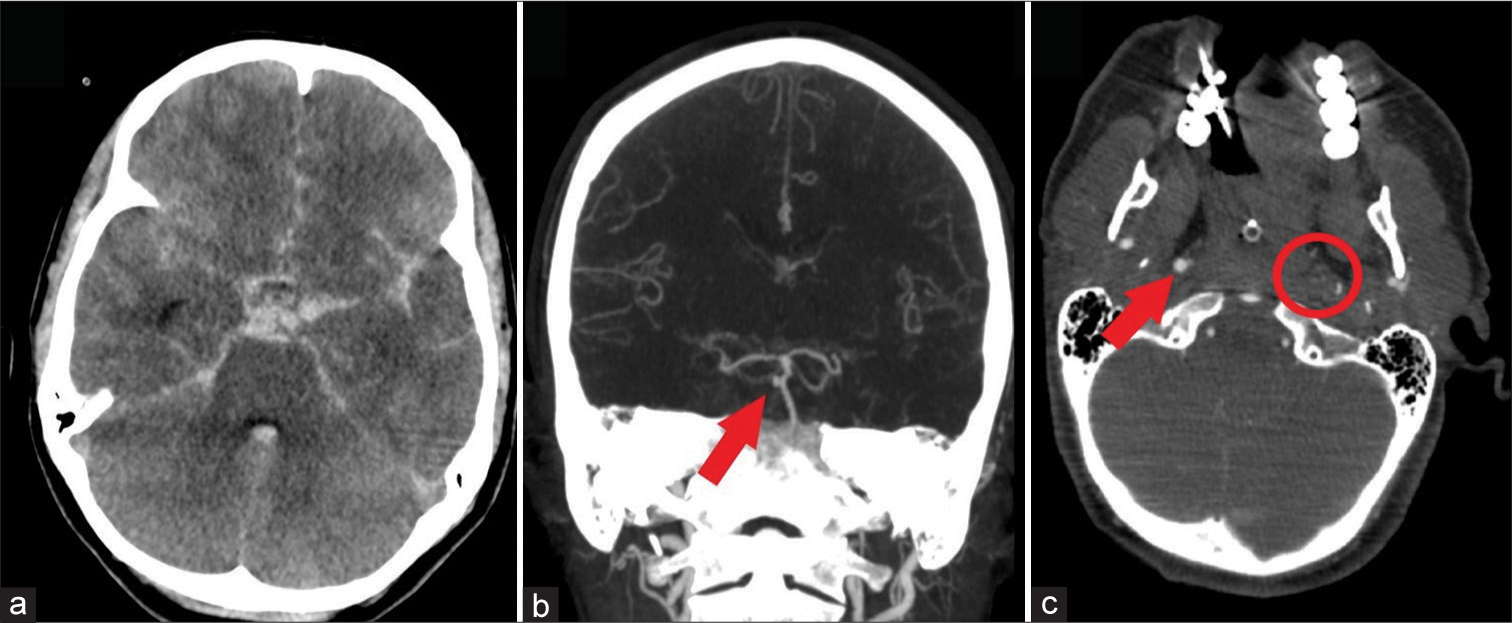
Figure 1:
(a) An axial image of the computed tomography (CT) scan at admission shows diffuse subarachnoid hemorrhage. A CT angiography was performed subsequently (b) revealing a saccular basilar trunk aneurysms (arrow). Furthermore, the absence of contrast was noted in the distal extracranial segment of the left internal carotid artery (encircled), whereas the right internal carotid artery was clearly visible (arrow).
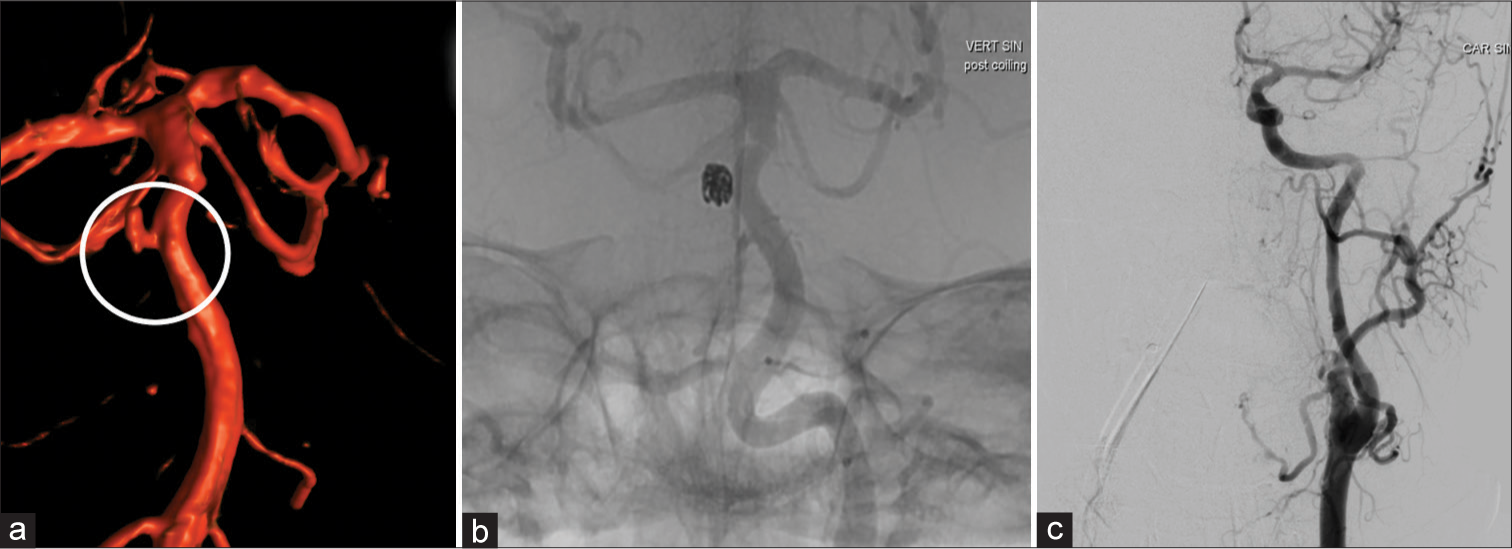
Figure 2:
The digital subtraction angiography images from the coiling procedure are shown. The 3D reconstruction of the vertebrobasilar arterial system illustrates the saccular basilar trunk aneurysms (encircled) in (a). Complete obliteration of the aneurysm was accomplished by bare coiling (b). In the same session, the left internal carotid artery was assessed to evaluate the left-sided carotid artery dissection. As can be seen in (c), the dissection is no longer visible and there is no sign of obstruction or stenosis of the left internal carotid artery.
After the procedure, the patient’s GCS was 5 (E1M3 (right side) V1). A CT scan of the head showed multiple hypodense areas within the territory of the left ICA suggestive of ischemia. This was probably due to the dissection of the left ICA [
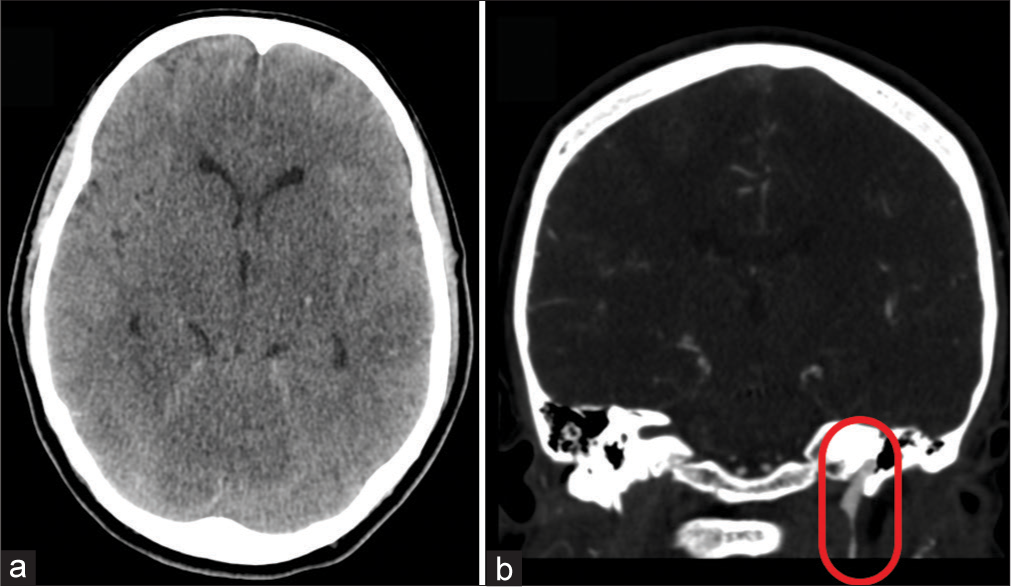
Figure 3:
An axial slide of the noncontrast-enhanced computed tomography (CT) scan of the brain shows a cortical hypodensity within the left temporal lobe (a). The gray-white matter differentiation is difficult to assess due to the subarachnoid hemorrhage, but might be slightly reduced. Furthermore, the bilateral deep nuclei like basal ganglia and thalami seem to appear less distinctively. Both might suggest hypoperfusion or ischemia. The CT angiography (b) again revealed the mouse-tail appearance of the distal extracranial segment of the left internal carotid artery (oval), suggestive for recurrent severe stenosis of this segment due to a subintimal dissection.
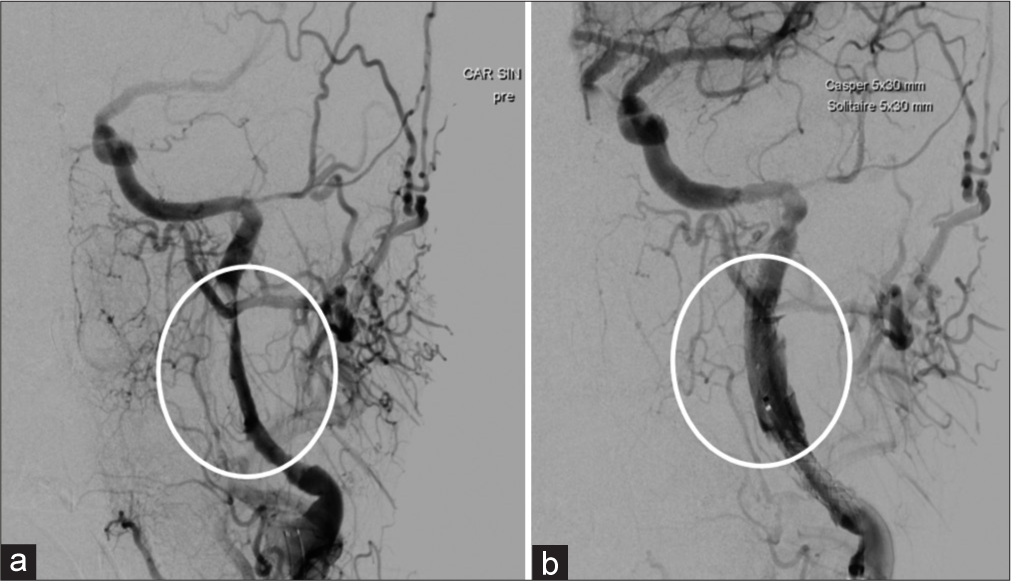
Figure 4:
These digital subtraction angiography images show the left internal carotid artery pre- and post-stent placement, in image a and b, respectively. Clearly, contrast filling is increasingly reduced from around halfway the extracranial segment of the internal carotid artery until its entrance into the petrosal bone. Following placement of two stents, the stenosis is redressed and internal carotid artery is filling accordingly.
After this procedure, the patient’s consciousness deteriorated to E1M2 (right side) V1 and repeated head CT scan showed progressive hypodensities. Antithrombotic medication was changed to ASA in combination with Prasugrel. The patient’s GCS deteriorated to 3 (E1M1V1) and a magnetic resonance imaging of the head was performed showing extensive and diffuse ischemia in the mesencephalon, bilateral deep nuclei, thalamus, and watershed areas [
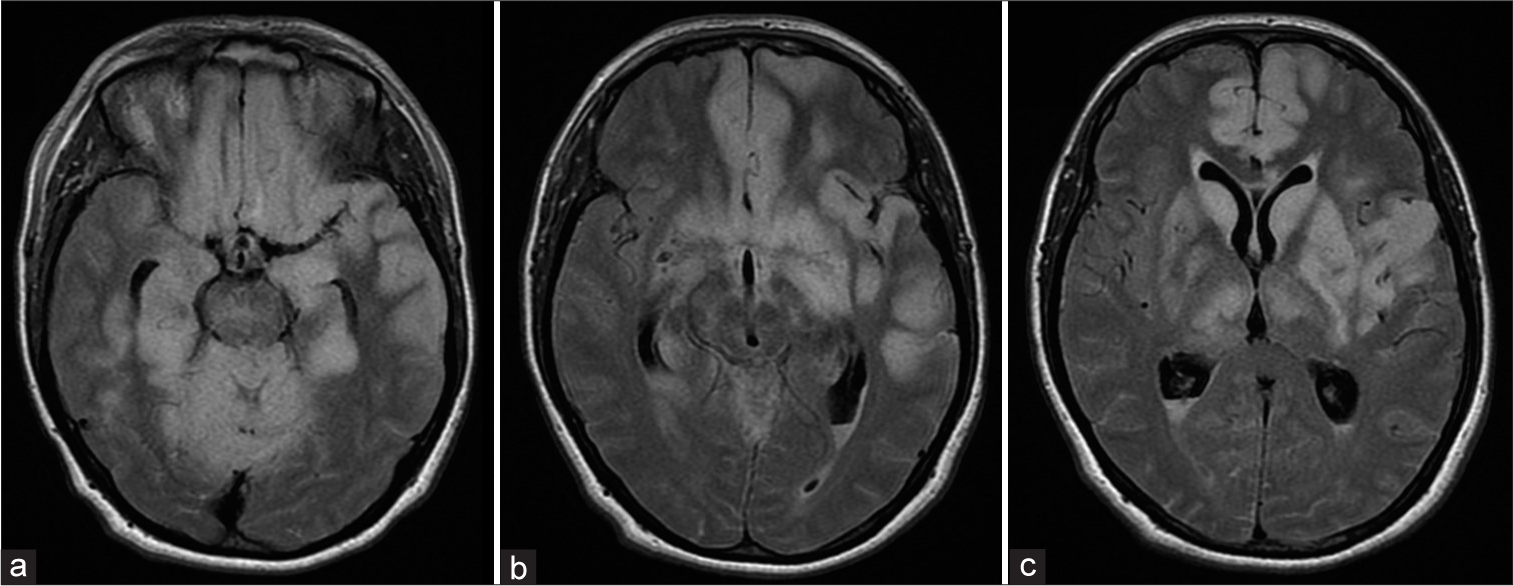
Figure 5:
(a-c) Axial FLAIR images of the magnetic resonance imaging depict hyperintensities within the mesencephalon, bilateral thalamic, and basal ganglia (left more pronounced than right). In addition, paramedian and lateral cortical hyperintensities within the frontal lobe (in particular on the left side) and in the left temporal lobe are visible. In combination with the findings on diffusion-weighted images and the apparent diffusion coefficient images (not shown), these hyperintensities were diagnosed as ischemia within these areas. The subintimal dissection of the left-sided internal carotid artery is probably the reason for the more pronounced left-sided location of the ischemic lesions.
DISCUSSION
To the best of our knowledge, this is the first report on the simultaneous occurrence of a CAD and BTA. Moreover, there seems to be a direct relation with the preceding trauma, and the patient suffered from a known underlying vasculitis. In this discussion, we describe these various conditions and their mutual relations, and aim to grasp how they may have interacted in this patient.
CADs
The reported annual incidence of CADs is around 2.6/100,000.[
Despite the etiology of CADs is considered spontaneous in most cases, a trauma or other mechanical triggers, including sports activities, are reported in up to 40% of cases.[
BTA
Of all IAs, saccular BTAs comprise <0.5%.[
CAD and IAs
In an attempt to evaluate whether the concomitant incidence of CADs and IA is common, we have reviewed the literature on this topic. Based on our findings, there are two clinical studies and few case reports describing either the presence of an IA in a patient with CAD or the presence of a CAD in a SAH patient [
In their series of 164 patients with a CAD, Schievink et al. described an incidence rate of 5.5% for IA.[
Based on the increased incidence of their mutual concomitant occurrence, a shared pathogenesis may be suggested. This hypothesis is supported by the fact that both vascular entities share common risk factors, such as hypertension and smoking, and are associated with similar connective tissue and vascular disorders.[
Based on these mutual associations and epidemiological findings, the hypothesis of a shared pathogenesis seems justified. However, the number of clinical studies on this topic is restricted and study sizes limited, reducing their clinical value. The restricted number of studies as well as case reports may on itself suggest that the concomitant incidence of CADs and IAs is uncommon. Finally, the development of CADs as well as the rupture of an IA induces instant hemodynamic alterations, including altered hemodynamic flow patterns and blood pressure increase.[
HSP and CAD or IA
Since our patient was known with HSP, we also considered a contribution of HSP in this case. HSP is a leukocytoclastic vasculitis involving the microvasculature and predominantly affecting children.[
Application to our case
As we now comprehend relevant characteristics and mutual interactions of the involved disorders in our case, we aim to understand the sequence of events by evaluating the clinical course and radiological findings. The clinical course was initiated following a trauma which suggests a causal relation. As mentioned, a preceding trauma is frequently reported in CADs, whereas it has rarely been related to the rupture of an IA.[
Treatment and prognosis
The clinical course of spontaneous and posttraumatic CAD is often benign with complete disappearance in most cases.[
The clinical course in our case was further complicated by the initial cardiac arrest. Our patient developed extensive and severe cerebral ischemia despite antithrombotic therapy. The ischemic lesions were most apparent in territory of the left-sided ICA, potentially related to the dissection of the left ICA. The ischemic lesions within the right ICA territory, vertebrobasilar territory, and watershed areas were attributed to hypoperfusion during the cardiac arrest and a possible contribution of delayed cerebral ischemia and vasospasms in the course of the SAH.
CONCLUSION
CAD and BTAs are both associated with vascular fragility. Our case is nevertheless the first report on their concomitant occurrence. The previous studies have suggested an increased incidence of IAs in patients with a CAD and vice versa. As the number of these studies and reports is limited, a mutual increased incidence and potential shared pathogenesis seem speculative. In our case, our inquiry suggests that a trauma leads to a left-sided CAD with secondary rupture of a saccular BTA.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akiyama Y, Moritake K, Miyazaki T, Nagai H. Subarachnoid hemorrhage in the presence of both intracranial dissecting and saccular aneurysms-two case reports. Neurol Med Chir (Tokyo). 2007. 47: 65-9
2. Brandt T, Morcher M, Hausser I. Association of cervical artery dissection with connective tissue abnormalities in skin and arteries. Front Neurol Neurosci. 2005. 20: 16-29
3. Brandt T, Hausser I, Orberk E, Grau A, Hartschuh W, Anton-Lamprecht I. Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol. 1998. 44: 281-5
4. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008. 4: 34-42
5. Cummings TJ, Johnson RR, Diaz FG, Michael DB. The relationship of blunt head trauma, subarachnoid hemorrhage, and rupture of pre-existing intracranial saccular aneurysms. Neurol Res. 2000. 22: 165-70
6. Cutfield NJ, Wilson JL, Zwi LJ, Snow BJ. Internal carotid artery dissection, cerebral aneurysms and thin basement membrane nephropathy. J Neurol. 2008. 255: 1973-4
7. deSouza RM, Shah M, Koumellis P, Foroughi M. Subarachnoid haemorrhage secondary to traumatic intracranial aneurysm of the posterior cerebral circulation: Case series and literature review. Acta Neurochir (Wien). 2016. 158: 1731-40
8. DuBose J, Recinos G, Teixeira PG, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: Expanding experience. J Trauma. 2008. 65: 1561-6
9. Esposito G, Sabatino G, Lofrese G, Albanese A. Carotid artery dissection-related intracranial aneurysm development. Neurosurgery. 2012. 70: E511-5
10. Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MD, Rinkel GJ. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: A systematic review and meta-analysis. JAMA Neurol. 2019. 76: 588-97
11. Galyfos G, Filis K, Sigala F, Sianou A. Traumatic carotid artery dissection: A different entity without specific guidelines. Vasc Specialist Int. 2016. 32: 1-5
12. Giannini N, Ulivi L, Maccarrone M, Montano V, Orlandi G, Ferrari E. Epidemiology and cerebrovascular events related to cervical and intracranial arteries dissection: The experience of the city of Pisa. Neurol Sci. 2017. 38: 1985-91
13. Hall A, O’Kane R. The management of hypertension in preaneurysmal treatment subarachnoid hemorrhage patients. World Neurosurg. 2019. 125: 469-74
14. Hetland LE, Susrud KS, Lindahl KH, Bygum A. Henochschönlein purpura: A literature review. Acta Derm Venereol. 2017. 97: 1160-6
15. Higa T, Ujiie H, Kato K, Kamiyama H, Hori T. Basilar artery trunk saccular aneurysms: Morphological characteristics and management. Neurosurg Rev. 2009. 32: 181-91
16. Jamil O, Taussky P, Schmidt RH, Park MS. Fulminant vasculitis associated with extracranial dissections and occlusion, ischemic strokes, and aneurysm rupture: Case report and review of the literature. World Neurosurg. 2016. 91: 674.e7-11
17. Kadian-Dodov D, Gornik HL, Gu X, Froehlich J, Bacharach JM, Chi YW. Dissection and aneurysm in patients with fibromuscular dysplasia: Findings from the U.S. registry for FMD. J Am Coll Cardiol. 2016. 68: 176-85
18. Kaps M, Dorndorf W, Damian MS, Agnoli L. Intracranial haemodynamics in patients with spontaneous carotid dissection-transcranial Doppler ultrasound follow-up studies. Eur Arch Psychiatry Neurol Sci. 1990. 239: 246-56
19. Kwak JH, Choi JW, Park HJ, Chae EY, Park ES, Lee DH. Cerebral artery dissection: Spectrum of clinical presentations related to angiographic findings. Neurointervention. 2011. 6: 78
20. Löfberg M, Niemelä M, Pieninkeroinen I. Kaulavaltimon dissektoituma. Duodecim. 1989. 107: 1889-92
21. Majamaa K, Portimojarvi H, Sotanieml KA, Myilyia VV.editors. Familial aggregation of cervical artery dissection and cerebral aneurysm: To the editor. Stroke. 1994. 25: 1704-5
22. Marshman LA, Ball L, Jadun CK. Spontaneous bilateral carotid and vertebral artery dissections associated with multiple disparate intracranial aneurysms, subarachnoid hemorrhage and spontaneous resolution. Case report and literature review. Clin Neurol Neurosurg. 2007. 109: 816-20
23. Mazighi M, Saint Maurice JP, Rogopoulos A, Houdart E. Extracranial vertebral and carotid dissection occurring in the course of subarachnoid hemorrhage. Neurology. 2005. 65: 1471-3
24. Rautalin I, Korja M, Kaprio J. Smoking causes fatal subarachnoid hemorrhage: A case-control study of Finnish twins. Stroke. 2020. 51: 3018-22
25. Roth C, Kleffmann J, Bergmann C, Deinsberger W, Ferbert A. Ruptured cerebral aneurysm and acute bilateral carotid artery dissection in a patient with polycystic kidney disease and polycystic liver disease. Cerebrovasc Dis. 2013. 35: 590-1
26. Saliou G, Sacho RH, Power S, Kostynskyy A, Willinsky RA, Tymianski M. Natural history and management of basilar trunk artery aneurysms. Stroke. 2015. 46: 948-53
27. Schievink WI, Mokri B, Piepgras DG. Angiographic frequency of saccular intracranial aneurysms in patients with spontaneous cervical artery dissection. J Neurosurg. 1992. 76: 62-6
28. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001. 344: 898-906
29. Schievink WI, Limburg M, Oorthuys JW, Fleury P, Pope FM. Cerebrovascular disease in Ehlers-Danlos syndrome Type IV. Stroke. 1990. 21: 626-32
30. Schievink WI, Mokri B, Michels VV, Piepgras DG. Familial association of intracranial aneurysms and cervical artery dissections. Stroke. 1991. 22: 1426-30
31. Schlemm L, Nolte CH, Engelter ST, Endres M, Ebinger M. Cervical artery dissection after sports-an analytical evaluation of 190 published cases. Eur Stroke J. 2017. 2: 335-45
32. Steinmann J, Hartung B, Bostelmann R, Kaschner M, Karadag C, Muhammad S. Rupture of intracranial aneurysms in patients with blunt head trauma: Review of the literature. Clin Neurol Neurosurg. 2020. 199: 106208
33. Takeuchi S, Wada K, Sakakibara F, Mori K. A surgical case of paraclinoid carotid aneurysm associated with ipsilateral cervical internal carotid artery dissection. Neurol India. 2012. 60: 517-9
34. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36