- Neuroradiology Unit, Department of Biomedical Sciences and Morphological and Functional Imaging, University of Messina, Messina, Italy
- Section of Pathological Anatomy, Department of Human Pathology, University of Messina, Messina, Italy
- Section of Neurosurgery, Department of Biomedical Sciences and Morphological and Functional Imaging, University of Messina, Messina, Italy
- Section of Neurosurgery, Department of Experimental Biomedicine and Clinical Neurosciences (BIONEC), University of Palermo, Palermo, Italy
- Section of Neurosurgery, Department of Human Pathology, University of Messina, Messina, Italy
Correspondence Address:
Enricomaria Mormina
Section of Neurosurgery, Department of Biomedical Sciences and Morphological and Functional Imaging, University of Messina, Messina, Italy
DOI:10.4103/sni.sni_33_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Francesca Granata, Sergio Racchiusa, Enricomaria Mormina, Valeria Barresi, Giada Garufi, Giovanni Grasso, Francesco Maria Salpietro, Marcello Longo, Concetta Alafaci. Presurgical role of MRI tractography in a case of extensive cervicothoracic spinal ependymoma. 26-Apr-2017;8:56
How to cite this URL: Francesca Granata, Sergio Racchiusa, Enricomaria Mormina, Valeria Barresi, Giada Garufi, Giovanni Grasso, Francesco Maria Salpietro, Marcello Longo, Concetta Alafaci. Presurgical role of MRI tractography in a case of extensive cervicothoracic spinal ependymoma. 26-Apr-2017;8:56. Available from: http://surgicalneurologyint.com/surgicalint-articles/presurgical-role-of-mri-tractography-in-a-case-of-extensive-cervicothoracic-spinal-ependymoma/
Abstract
Background:Intramedullary spinal ependymoma is a tumor, hardly characterizable with conventional magnetic resonance (MR) imaging only. MR diffusion tensor imaging (DTI) with three-dimensional fiber-tracking reconstructions allows the evaluation of the relationship between neoplasm and white matter fiber tracts, being a powerful tool in presurgical planning. We present DTI findings in a case of a young female with an extensive cervicothoracic spinal ependymoma.
Case Description:The patient complained of a 2-month history of acute urinary retention, weakness and numbness on the lower limbs and the upper left limb. She underwent MR imaging that showed an extensive cervicothoracic spinal mass, difficult to characterize with conventional MR sequences. DTI showed peripherally displacement of fibers, without involvement of the spinal cord, findings consistent with an ependymoma. The patient underwent surgery with a complete resection “en bloc” of the lesion, which showed clear cleavage planes, as detected by DTI. Histopathological findings confirmed the diagnosis of ependymoma.
Conclusions:DTI is a useful tool in presurgical planning, helping in differentiating not infiltrating neoplasms, such as spinal ependymomas, from other infiltrative and more aggressive neoplasms, which are considered not resectable.
Keywords: Cervicothoracic tract, DTI, ependymoma, spinal cord
INTRODUCTION
Cervicothoracic spinal cord ependymomas are rare neoplasms, noninfiltrative lesions, characterized by a cleavage plane from the spinal cord and therefore, considered resectable. MRI is the gold standard in the diagnosis of spinal ependymomas. However, differential diagnosis between spinal ependymoma and infiltrating tumors, such as astrocytomas, may be a diagnostic dilemma on conventional magnetic resonance (MR) imaging.[
MR diffusion tensor imaging (DTI), is an MR technique that evaluates the diffusion of water molecules within the white matter fibers. Fiber-tracking (FT) algorithms allow reconstruction of three-dimensional (3D) images of white matter in the spinal cord, enabling the assessment of his involvement and evaluation of the relationship between the neoplasm and the white matter tracts, playing a helpful role in surgical planning.[
We present a case of a young female with an extensive cervicothoracic spinal ependymoma, in which DTI with 3D fiber-tracking reconstructions was a powerful tool for the diagnosis.
To our knowledge, there are only few reports that emphasize the role of MR diffusion tensor tractography with 3D fiber-tracking reconstructions in the assessment of cervicothoracic spinal ependymoma.
CASE REPORT
A 27-year-old woman with a 2-month history of acute urinary retention was admitted to our institution on February 2016. Her symptoms started with weakness and numbness on the left lower limb with progressive involvement of the upper left limb and the right lower limb.
Laboratory tests did not show any significant alterations. Patient underwent spine MR imaging.
MR evaluation was performed on a 1.5 Tesla MR Scanner (Philips Ingenia Philips, The Netherlands, Best). Morphological sequences included a TSE T2-weighted in axial, sagittal, and coronal plane (TR/TE = 3000/120 ms with a slice thickness of 3.5mm for sagittal and coronal planes and 6mm slice thickness for the axial one); a sagittal TSE T1-weighted (TR/TE = 500/9 ms with a slice thickness of 2.5mm) before and after gadolinium administration (15ml of Gadovist; 0.1 mmol per kilogram of body weight). Diffusion data were also acquired by using a DWIBS sequence (TR/TE = 3299/68 ms, 5 mm thickness, no interstice gap, b = 1000 s/mm2), and a DTI with 32 different diffusion gradient with a b = 1000 s/mm2 (TR/TE = 4299/91 ms, voxel size = 2 mm × 2 mm × 2 mm). ADC map was also created. No interslice gap was used in any of the abovementioned sequences. MR diffusion data for tractography were processed after motion correction and eddy current-induced distortion correction. Tractography and 3D rendering were performed with DSI Studio software (
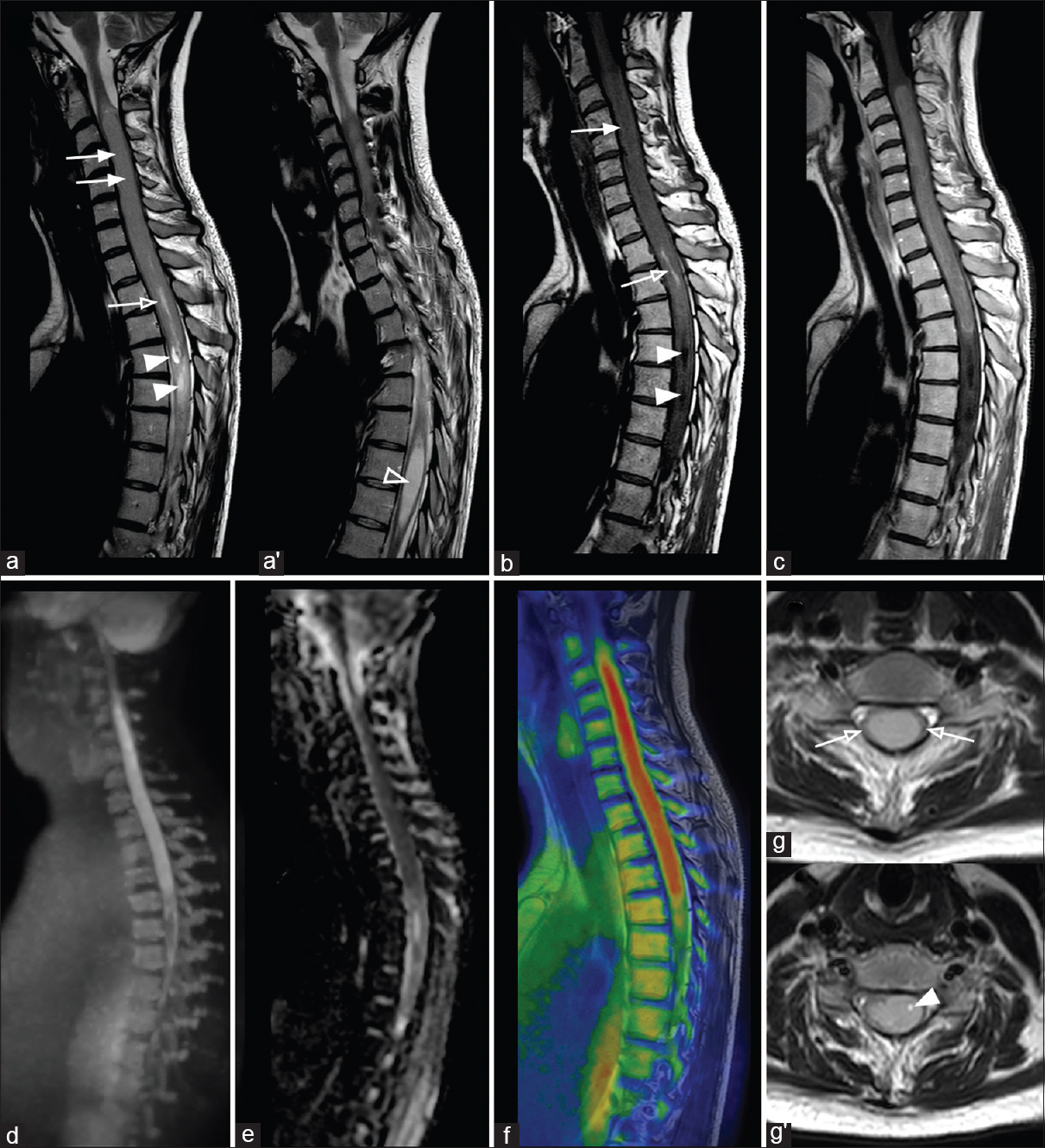
MR conventional imaging showed an extensive cervicothoracic spinal mass with longitudinal size of 145 mm and axial maximum size of 11 mm. The mass extended from C2 to T3 and was characterized by a solid part slightly hyperintense on T2-weighted images and hypointense on T1-weighted images; some cystic components and a small amount of late subacute bleeding inside the neoplasm were also detected (methemoglobin) [
Figure 1
Spinal cord MRI of a young patient affected by an extensive cervicothoracic spinal ependymoma. (a) Consecutive sagittal T2-weighted images show a cervicothoracic spinal ependymoma with a solid part (arrows), some cystic parts (arrowheads) and a small amount of late sub-acute bleeding inside the neoplasm (methemoglobin) (empty arrow). Note the syringomyelia at T6-T9 level (a’ – empty arrowhead). Note also caliber change of the spinal cord at C2-C3 level and moderate widening of spinal canal. (b) Sagittal noncontrast T1-weighted shows a cervicothoracic spinal ependymoma with a solid part (arrow), some cystic parts (arrowhead), and a small amount of late sub-acute bleeding inside the neoplasm (methemoglobin) (empty arrow). (c) Sagittal post-contrast T1-weighted image shows the cervicothoracic extension of ependymoma's solid part. (d) Sagittal DWIBS-MIP reconstruction, (e) sagittal ADC map and (f) fused T1-weighted/DWIBS images show a high restriction of diffusion a the level of ependymoma's solid part, respectively high, low and high (red) signal intensity. (g) Sagittal T2-weighted images show, at C6-C7 and (g’) C5 level, white matter peripheral displacement surrounding ependymoma (empty arrows) and a small intralesional cyst (arrowhead), respectively
Contrast-enhanced T1-weighted images showed a significant degree of lesion enhancement [
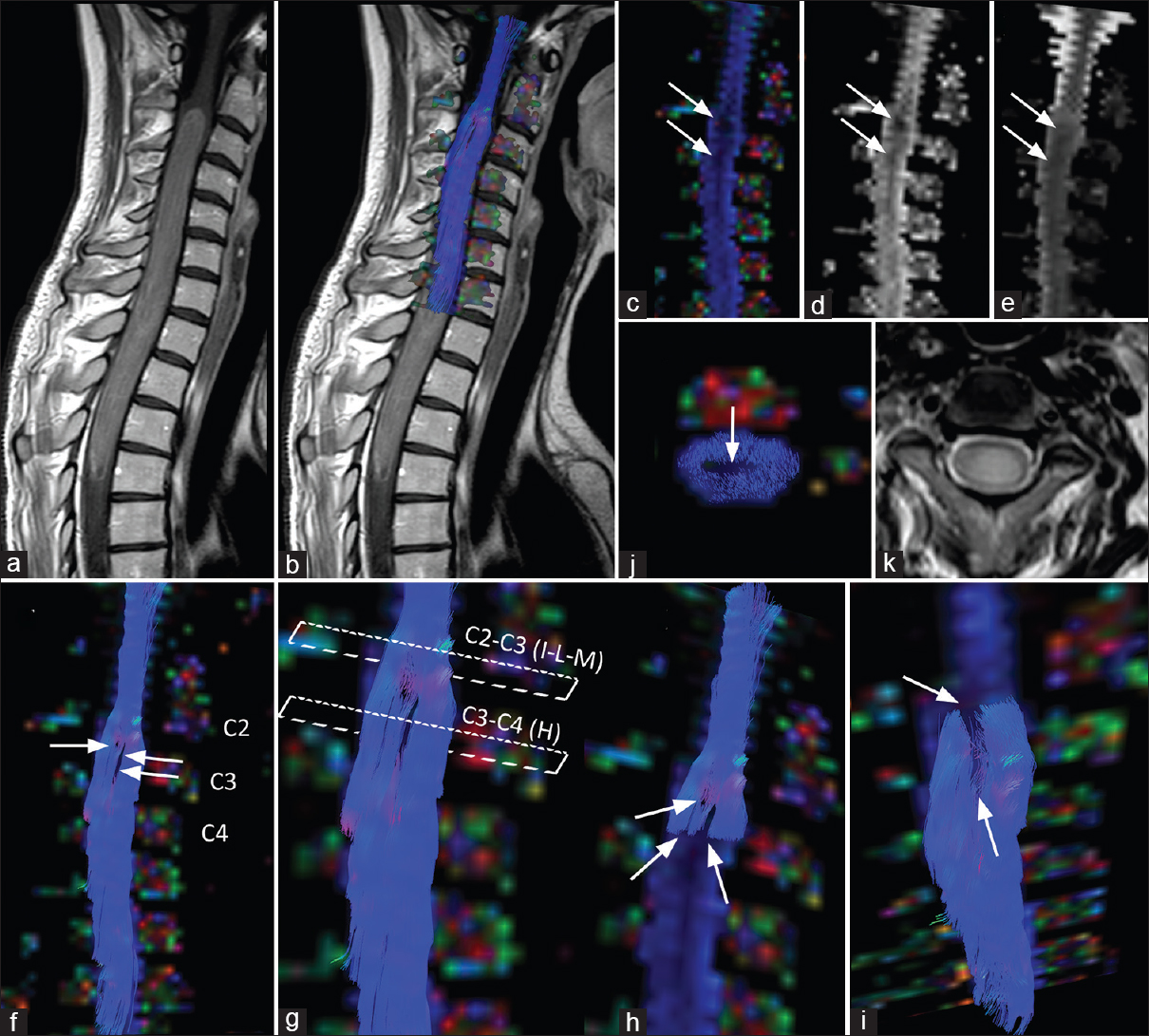
DTI FT of the spinal cord showed that neoplasm did not infiltrate the spinal cord with a disruption of axonal tracts. Moreover, DTI FT showed the sparing of white matter fiber tracts by the neoplasm and the peripheral displacement of fiber bundles [
Figure 2
Spinal cord MRI tractography of a young patient affected by spinal ependymoma. The panel shows no infiltration of the spinal cord but rather cord fibers peripherally displacement. (a and b) Sagittal post-contrast T1-weighted images, without (a) and with (b) spinal cord tractography overlayed, show the extension of ependymoma's compact part. Note the change in caliber of spinal cord at C2-C3 level. (c-e) Sagittal color-coded fractional anisotropy (FA) map (c), FA map, (d), and ADC map (e), show low intensity values (arrows) corresponding to the ependymoma, centrally placed. (f-i) Sagittal color-coded FA map with spinal cord tractography overlaid shows different point of fiber displacement (arrows), rather than infiltration, by ependymoma. Images h, i, and l were obtained by using a “virtual cut” of part of the spinal cord fibers obtained respectively at C3-C4 (h) and C2-C3. (i,j) levels. (l) Axial color-coded FA map with spinal cord tractography overlaid and (k) T2-weighted images at C2-C3 level shows white matter peripheral displacement surrounding ependymoma. Arrow indicates fibers lack in the central part of spinal cord, caused by a central position of the neoplasm
By using a manipulation of the 3D FT output data, we performed a “virtual cut” of part of the spinal cord fibers, obtained at C2-C3 and C3-C4 levels respectively [Figure
Surgical treatment
The patient underwent a C2–T5 laminectomy through a posterior approach. Once the dura was opened, the spinal cord appeared compressed, bulging through the dural opening. A central myelotomy D2–D4 was performed. After the myelotomy, a mass with a caudal and cranial identifiable cleavage plane was found, as previously shown by tractography which was used during the presurgical planning.
The tumor was brownish colored, soft, highly vascularized, and due to the cleavage plane from the spinal cord was easily removed “en block.” The length of the tumor was 145 mm. Optimal decompression of the spinal cord was achieved. Electrophysiological monitoring of motor, somatosensory evoked potentials and D-wave was performed. The patient underwent C3–D2 stabilization with a titanium Vertex Medtronic system.
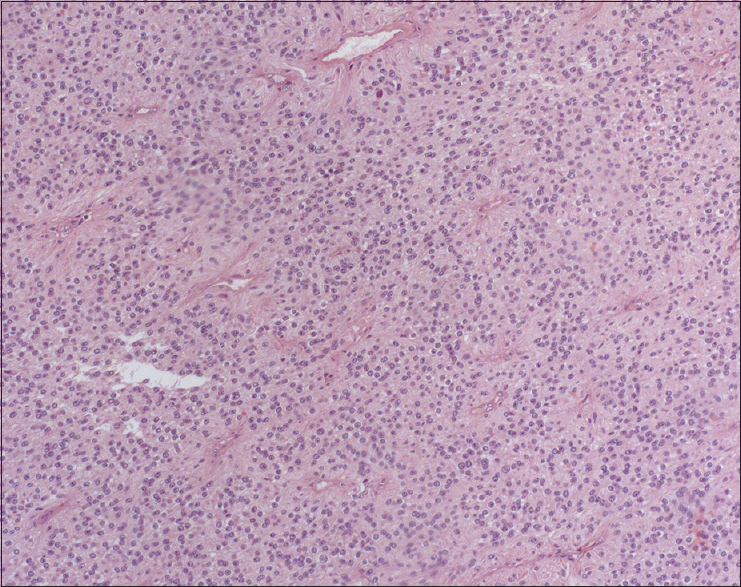
Histopathological findings
Sections of the tumor microscopically showed a glial neoplasm characterized by a lot of cells with rounded nuclei, perivascular pseudorosette formations, ependymal canals, and ependymal rosettes [
Few foci of coagulative necrosis were also detected. Immunohistochemical analysis showed neoplastic cells positive for GFAP, S100, and EMA (dot-like), and negative for IDH1, R132H, and p53. The Ki-67 labeling index was 2%. The final pathologic diagnosis was an ependymoma WHO grade II.
DISCUSSION
Intramedullary ependymomas are spinal cord tumors arising from the ependymal cells of the central canal, and are characterized by a cleavage plane from the spinal cord due to a concentric growth.[
MRI is the “gold standard” for the noninvasive differential diagnosis between these two intramedullary tumors because it has a key role in distinguishing noninvasive neoplasms, such as ependymomas, from diffusely infiltrating tumors, such as astrocytomas. Nonetheless, a proper diagnosis based only on conventional MRI is often challenging. Advanced MRI techniques, such as DTI FT, have been widely used in the last decade to provide further useful information in tumor characterization and in presurgical planning. DTI is an MR technique which evaluates the diffusion of water molecules within the white matter fibers. By means of this evaluation, it is possible to obtain FT data 3D reconstruction of spinal cord white matter, and to investigate spinal cord structural damage and/or tumor infiltration. Interestingly, only few reports emphasize the role of DTI in the assessment of cervicothoracic tumors and its presurgical role.[
Setzer et al. in their retrospective study, investigated the predictive value of DTI imaging in the assessment of intramedullary spine tumor resection compared to intraoperative surgical findings, evaluating the fiber course in different types of spinal tumors with DTI tractography. This preliminary study suggested that DTI tractography was able to predict the resectability of the lesion.[
Ducreux et al. showed DTI findings in five cases of spinal cord astrocytoma, suggesting that this technique could be used for assessment of solid astrocytomas due to the visualization of irregular course of “warped” fibers.[
Our case showed DTI findings in a cervicothoracic intramedullary ependymoma with a large extension from C2 to T3. Despite the size of the lesion, and the late subacute bleeding component in his context, DTI easily showed different points of fibers displacement rather than infiltration. DTI was able to show not only the white matter fibers peripherally displaced but also the integrity of these fibers and the lack of them in the central portion of spinal cord because of neoplasm's central position. DTI was helpful to make the correct diagnosis of intramedullary ependymoma, which was histologically confirmed after surgery, and showed during the presurgical planning the possibility of obtaining a cleavage plane during surgery.
There are few reports that clearly explain the tractographic semeiology of intramedullary spinal cord neoplasm, especially when a mass involves such a long cervical and thoracic segment. In our case, we find DTI FT helpful in differential diagnosis between infiltrative and noninfiltrative masses and in presurgical planning. Therefore, we suggest the use of this advanced MRI technique, together with the conventional MRI, to perform a more accurate evaluation of any spinal cord tumor.
CONCLUSIONS
DTI findings reported in this case suggest that this technique can be useful in the differentiation of spinal ependymomas from other more aggressive lesions, also in extensive cases, playing a strong role not only in the diagnosis but also in neurosurgical planning, for both biopsy or resection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chimelli L. Tumors and tumor like lesions of the spine and spinal cord. Neuroimaging Clin N Am. 2001. 11: 79-110
2. Choudhri AF, Whitehead MT, Klimo PJr, Montgomery BK, Boop FA. Diffusion tensor imaging to guide surgical planning in intramedullary spinal cord tumors in children. Neuroradiology. 2014. 56: 169-74
3. Ducreux D, Fillard P, Facon D, Ozanne A, Lepeintre JF, Renoux J. Diffusion tensor magnetic resonance imaging and fiber tracking in spinal cord lesions: Current and future indications. Neuroimaging Clin N Am. 2007. 17: 137-47
4. Ducreux D, Lepeintre JF, Fillard P, Loureiro C, Tadié M, Lasjaunias P. MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol. 2006. 27: 214-6
5. Egger K, Hohenhaus M, Van Velthoven V, Heil S, Urbach H. Spinal diffusion tensor tractography for differentiation of intramedullary tumor-suspected lesions. Eur J Radiol. 2016. 85: 2275-80
6. Lerner A, Mogensen MA, Kim PE, Shiroishi MS, Hwang DH, Law M. Clinical applications of diffusion tensor imaging. World Neurosurg. 2014. 82: 96-109
7. Phillips NS, Sanford RA, Helton KJ, Boop FA, Zou P, Tekautz T. Diffusion tensor imaging of intraaxial tumors at the cervicomedullary and pontomedullary junctions. Report of two cases. J Neurosurg. 2005. 103: 557-62
8. Radek M, Wiśniewski K, Grochal M, Jastrzębski K, Gębski P, Snopkowska-Wiaderna D. Spinal cord diffusion tensor tractography as a diagnostic tool in difficult cases of spinal cord intramedullary tumours. Neurol Neurochir Pol. 2013. 47: 74-9
9. Setzer M, Murtagh RD, Murtagh FR, Eleraky M, Jain S, Marquardt G. Diffusion tensor imaging tractography in patients with intramedullary tumors: Comparison with intraoperative findings and value for prediction of tumor resectability. J Neurosurg Spine. 2010. 13: 371-80
10. Thurnher MM, Law M. Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magn Reson Imaging Clin N Am. 2009. 17: 225-44
11. Van Goethem JW, van den Hauwe L, Ozsarlak O, Ozsarlak O, De Schepper AM, Parizel PM. Spinal tumors. Eur J Radiol. 2004. 50: 159-76
12. Vargas MI, Delavelle J, Jlassi H, Rilliet B, Viallon M, Becker CD. Clinical applications of diffusion tensor tractography of the spinal cord. Neuroradiology. 2008. 50: 25-9
13. Liu X, Tian W, Kolar B, Hu R, Huang Y, Huang J. Advanced MR diffusion tensor imaging and perfusion weighted imaging of intramedullary tumors and tumor like lesions in the cervicomedullary junction region and the cervical spinal cord. J Neurooncol. 2014. 116: 559-66
14. Yeh F, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WI. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013. 8: e80713-