- Department of Radiology, Fundación Santa Fe de Bogotá, Bogota, Colombia
- Department of Medicine, Santa Casa de São Paulo Medical School, São Paulo, Brazil
- Department of Medicine, San Francisco School of Medicine, San Francisco, California, United States
- Department of Medicine, University of California, Riverside School of Medicine, Riverside, California, United States
- Department of Surgery, Massachusetts General Hospital, Boston, Massachusetts, United States
- Department of Internal Medicine, Rutgers New Jersey Medical School, Newark, United States
- Department of Medicine, Fundacion Universitaria de Ciencias de la Salud, Bogotá, Colombia
- Department of Nuclear Medicine, Fundación Universitaria Sanitas, Bogotá, Colombia
- Department of Surgery, Cantonal Hospital of St. Gallen, St. Gallen, Switzerland.
Correspondence Address:
Andres Felipe Herrera Ortiz, Fundación Santa Fe de Bogotá, Bogota, Colombia.
DOI:10.25259/SNI_190_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andres Felipe Herrera Ortiz1, Enrico Stefano Suriano2, Yasmin Eltawil3, Manraj Sekhon4, Anthony Gebran5, Mateo Garland6, Nury Tatiana Rincón Cuenca7, Tatiana Cadavid8, Bassel Almarie9. Prevalence and risk factors of unruptured intracranial aneurysms in ischemic stroke patients – A global meta-analysis. 30-Jun-2023;14:222
How to cite this URL: Andres Felipe Herrera Ortiz1, Enrico Stefano Suriano2, Yasmin Eltawil3, Manraj Sekhon4, Anthony Gebran5, Mateo Garland6, Nury Tatiana Rincón Cuenca7, Tatiana Cadavid8, Bassel Almarie9. Prevalence and risk factors of unruptured intracranial aneurysms in ischemic stroke patients – A global meta-analysis. 30-Jun-2023;14:222. Available from: https://surgicalneurologyint.com/surgicalint-articles/12389/
Abstract
Background: Unruptured intracranial aneurysms (UIAs) have an estimated global prevalence of 2.8% in the adult population; however, UIA was identified among more than 10% of ischemic stroke patients. Many epidemiological studies and reviews have pointed to the presence of UIA among patients with ischemic stroke; yet, the extent of this association is not fully known. We performed a systematic review and meta-analysis to determine the prevalence of UIA in patients admitted to hospitals with ischemic stroke and transient ischemic attack (TIA) at both global and continental levels and evaluate factors associated with UIA in this population.
Methods: We identified, in five databases, all studies describing UIA in ischemic stroke and TIA patients between January 1, 2000, and December 20, 2021. Included studies were of observational and experimental design.
Results: Our search yielded 3581 articles of which 23 were included, with a total of 25,420 patients. The pooled prevalence of UIA was 5% (95% confidence interval [CI] = 4–6%) with stratified results showing 6% (95% CI = 4–9%), 6% (95% CI = 5–7%), and 4% (95% CI = 2–5%) in North America, Asia, and Europe, respectively. Significant risk factors were large vessel occlusion (odds ratios [OR] = 1.22, 95% CI = 1.01–1.47) and hypertension (OR = 1.45, 95% CI = 1.24–1.69), while protective factors were male sex (OR = 0.60, 95% CI = 0.53–0.68) and diabetes (OR = 0.82, 95% CI = 0.72–0.95).
Conclusion: The prevalence of UIA is notably higher in ischemic stroke patients than the general population. Physicians should be aware of common risk factors in stroke and aneurysm formation for appropriate prevention.
Keywords: Computed tomography angiography, Ischemic stroke, Magnetic resonance angiography, Prevalence, Adults, Unruptured intracranial aneurysm
INTRODUCTION
Unruptured intracranial aneurysms (UIAs) have an estimated global prevalence of 2.8% in the adult population.[
Many epidemiological studies and reviews have pointed to the presence of UIA among patients with ischemic stroke;[
MATERIALS AND METHODS
Search strategy
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[
A search strategy consisting of 14 MeSH terms was developed in MEDLINE. The keywords for the search strategy were ((“Intracranial Aneurysm”[Mesh])) AND (“Stroke”[Mesh] OR “Embolic Stroke”[Mesh] OR “Thrombotic Stroke”[Mesh] OR “Ischemic Stroke”[Mesh] OR “Infarction, Middle Cerebral Artery”[Mesh] OR “Infarction, Anterior Cerebral Artery” [Mesh] OR “Stroke, Lacunar”[Mesh] OR “Transitory ischemic attack”) OR “Cerebrovascular accident”) OR “Cerebral Infarction”) OR “Brain Infarction”) OR “Acute Ischemic Stroke”) AND “Prevalence”[Mesh].
To locate additional relevant publications, we identified references from the articles that fit our inclusion criteria using a snowball approach. We also hand-searched five additional preselected, high-impact neurosurgery, and neurointerventional radiology journals: Journal of Neurology, Neurosurgery and Psychiatry, American Journal of Neuroradiology, Journal of Neurosurgery, and World Neurosurgery.
Eligibility criteria
We included observational studies (cohort, cross-sectional, case–control, and case series) and clinical trials in our search. The population of interest included patients aged ≥18 years that were admitted to the hospital for having an ischemic stroke or TIA but had no known predisposing genetic or autoimmune diseases. The identified exposure was UIA detected by imaging modalities (computed tomography, computed tomography angiography, magnetic resonance imaging, magnetic resonance angiography, or fluoroscopy angiography) in the population of interest.
We excluded studies that were determined to be of poor quality, according to the National Institutes of Health (NIH) Quality Assessment tool, and those lacking sufficient information to calculate prevalence. We also excluded study designs, such as reviews, editorials, commentaries, and case reports. In addition, if multiple studies used the same dataset or cohort, only the study with the largest sample size was included.
Study selection process
Articles identified by the search strategy were imported into the reference management software, Mendeley, version 1.19.5/2019 (London, United Kingdom). Duplicates were deleted and the remaining articles were screened by title and abstract for inclusion. For articles that passed the initial screen and for those where titles and abstracts were not sufficient to make a decision, full texts were retrieved and further assessed for final inclusion. Manuscripts not written in English were translated. Two investigators carried out the entire process independently and disagreements in the study selection were resolved through consensus.
Data collection and missing data
All of the data were extracted into a preconceived and standardized form in Microsoft Excel (Office 365: Microsoft, Redmond, Washington, United States).
The following qualitative data were extracted from each study: authors, year of publication, country of publication, study type, number of patients with ischemic stroke or TIA, number of patients with UIA, mean age, location of aneurysms, mean aneurysm size, and imaging method used to detect the aneurysm. The qualitative and quantitative data were extracted by all investigators and reviewed by two independent investigators. For the primary outcome, the numerical data extracted from each study included the total number of patients with ischemic stroke or TIA, number of patients with UIA, and subgroups according to continent. For the secondary outcome, the data extracted included first author, year of publication, number of exposed cases, exposed controls, unexposed cases, and unexposed controls. In the case of missing data or lack of relevant information in studies, authors were contacted.
Outcome variables
The primary outcome was the prevalence of UIA among patients with ischemic stroke and TIA, which was assessed globally and by continent. The secondary outcome was associated factors related to UIA in patients with stroke and TIA. Associated factors included were sex, size of occluded vessel (large or small), hypertension, smoking, diabetes, dyslipidemia, stroke in anterior/posterior circulation, and atrial fibrillation.
Risk of bias
Quality assessment of the included studies was evaluated by the NIH Study Assessment Tools. Each study was assessed based on eight to fourteen questions, according to study type. After assessment, studies were given a rating of good, fair, or poor quality. A “good” study has the least risk of bias, a “fair” one is susceptible to some bias that is not sufficient to invalidate its results, and a “poor” study holds significant bias.
Statistical analysis
The software STATA (StataCorp LLC, College Station, Texas, United States) was used to perform all statistical analyses in this study.
For the primary outcome, we measured prevalence of UIA in stroke and TIA patients using the following formula:
P = Prevalence; N = Total sample size of patients with ischemic stroke or TIA.
*Population of interest = Patients with ischemic stroke or TIA.
The results of the primary outcome were expressed as relative frequencies and pooled in a quantitative synthesis to determine the overall effect. Heterogeneity among studies was assessed using Cochran’s Q tests and I2 statistics, with Cochran’s Q < 0.05 and I2 > 50% indicating substantial heterogeneity.[
To account for patients’ characteristics and risk factors that might explain differences in the prevalence of UIA in the stroke/TIA population, we calculated OR using a 2 × 2 table for all the variables of the secondary outcomes, then we pooled the results in a quantitative synthesis. Heterogeneity for these differences was assessed using Cochran’s Q test and I2 test.
RESULTS
Study selection and characteristics
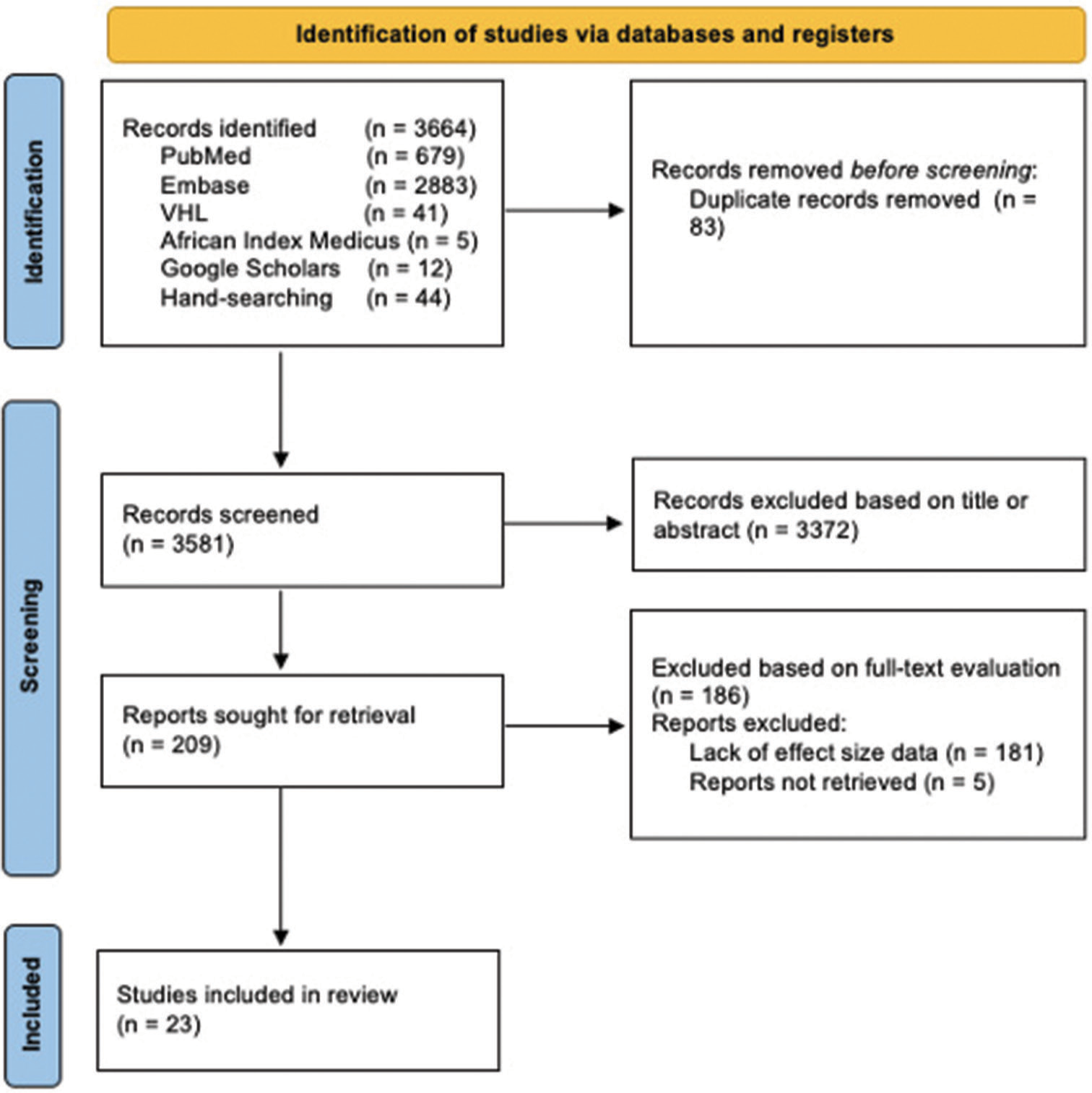
Our database search identified a total of 3664 articles. After excluding 3372 articles based on relevance to our primary objective and removing 83 duplicates, 209 articles remained. Of these, we filtered 186 articles because they failed to provide sufficient information regarding effect size or could not be found. Ultimately, we included 23 articles with a total sample size of 25,420 patients, spanning 13 different countries [
Among the studies included in this systematic review and meta-analysis, 22 were observational (15 retrospective, six prospective, and one descriptive) and one was a randomized clinical trial. The highest proportion of articles was from the United States (n = 8) and South Korea (n = 5). The remaining articles were from China, Japan, Taiwan, Singapore, Thailand, Germany, Greece, Italy, the United Kingdom, the Netherlands, and Canada. China and Japan alone contributed vastly to our final sample size, accounting for approximately 13,000 subjects.
For each of the 23 studies included, the items evaluated were first author, year of publication, country of publication, study type, number of patients with UIA, sample size, mean age, location of UIA, mean UIA size, and imaging modality [
Risk of bias
The risk of bias was assessed according to the NIH Quality Assessment Tool.[
Prevalence of UIA in stroke and TIA patients
Global prevalence
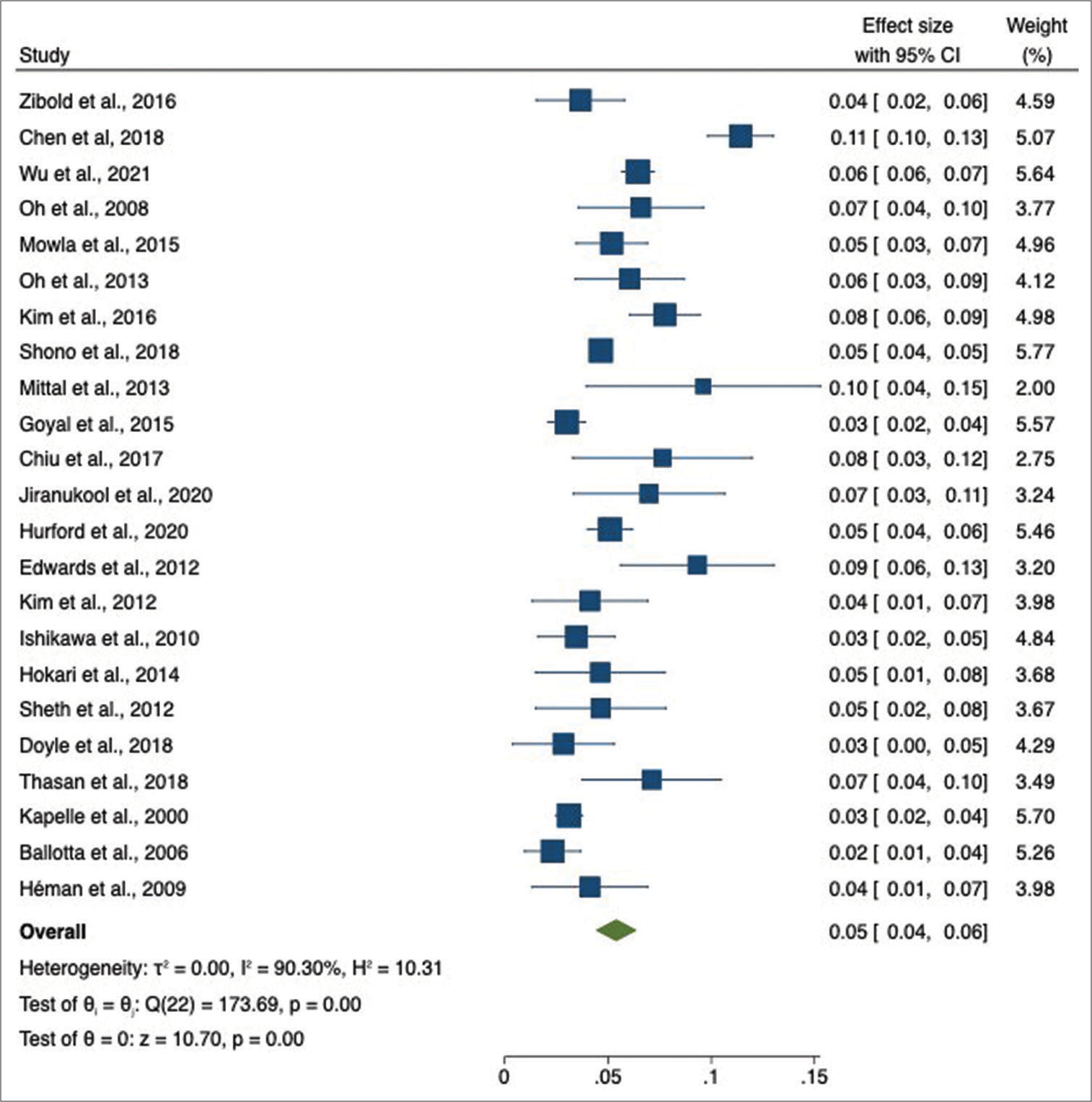
Pooled estimates of UIA prevalence were calculated for 23 studies totaling 25,420 stroke and TIA patients. Globally, the pooled prevalence was 5% (95% CI = 4–6%). Heterogeneity was statistically significant, with I2 = 90.3% and Cochran’s Q < 0.001 [
Figure 2:
Forest plot of unruptured intracranial aneurysms global prevalence in patients with ischemic stroke and transient ischemic attacks. The blue squares represents the prevalence of UIA in ischemic stroke patients. The lateral lines represents the confidence interval. The green rectangle represents the pool estimate of the prevalence in all studies. CI: Confidence interval.
Prevalence by continent
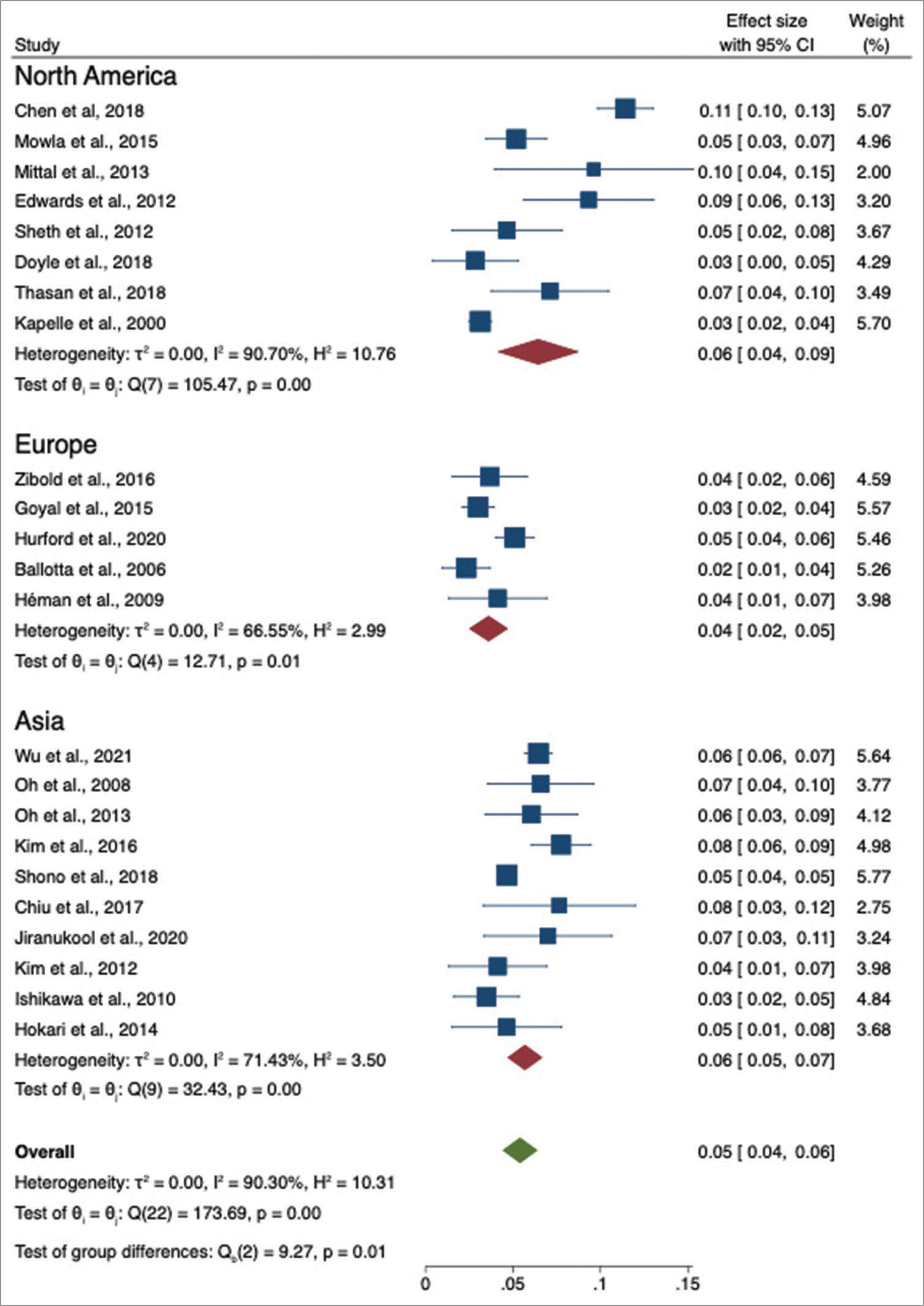
Pooled estimates of UIA prevalence were stratified by continent. Our study shows results from North America, Europe, and Asia. No data was available for Africa, South America, or Oceania. In North America, data from eight studies with a total of 5976 patients showed a pooled prevalence of 6% (95% CI = 4–9%) with substantial heterogeneity (I2 = 90.7%) [
Figure 3:
Forest plot of prevalence of unruptured intracranial aneurysms in patients with ischemic stroke or transient ischemic attacks by continent. The blue squares represents the prevalence of UIA in ischemic stroke patients in that study. The lateral lines represents the confidence interval. The red rectangle represents the pooled prevalence for that continent. The green rectangle represents the overall prevalence of all continents.
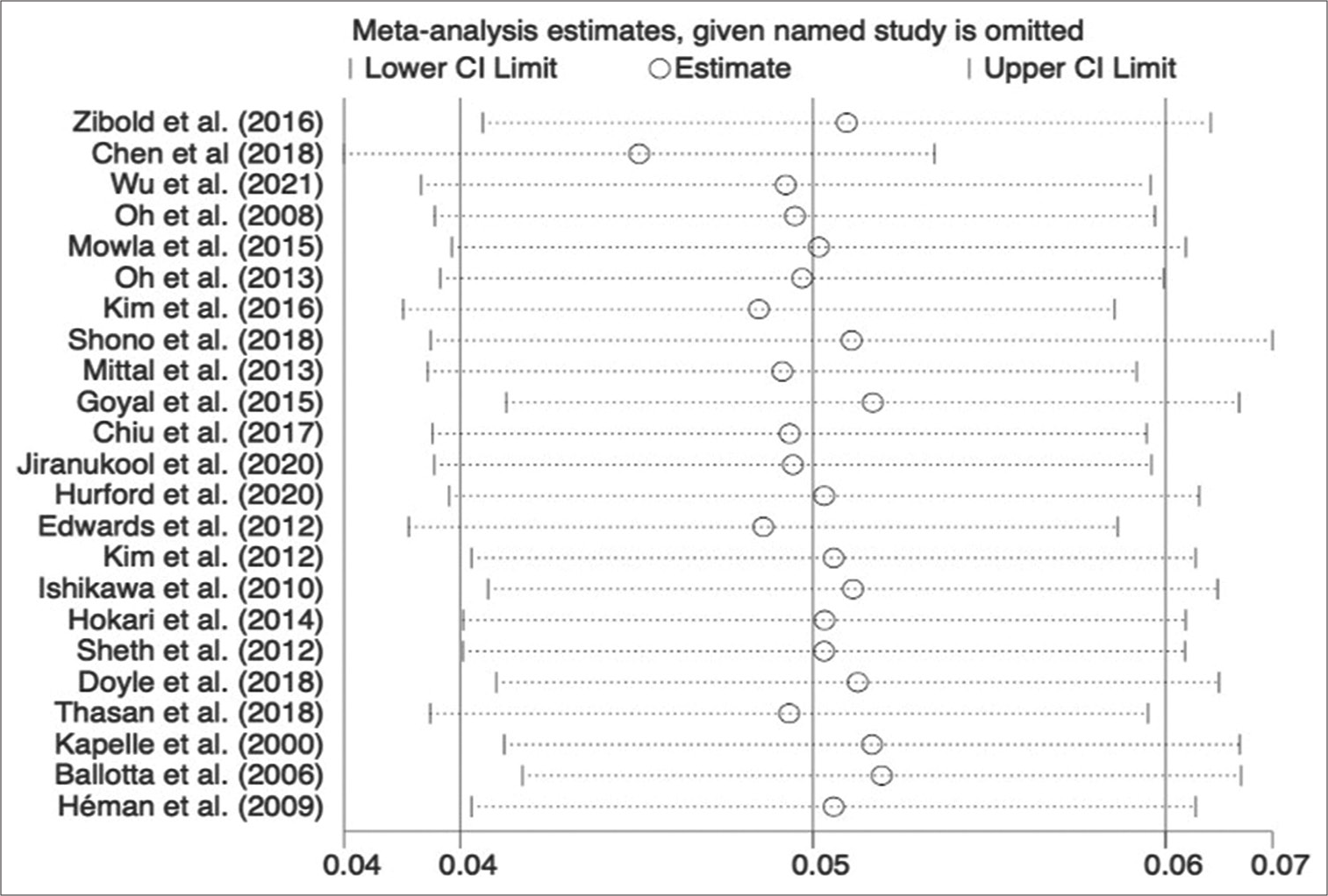
Sensitivity analysis and publication bias
A sensitivity analysis was performed to measure the effect of each study on the pooled estimate, revealing no change in the direction of the results [
Figure 4:
Sensitivity analysis of the prevalence of unruptured intracranial aneurysms in patients with ischemic stroke or transient ischemic attacks. The white circle represents the overall prevalence when removing the study that is on the left side. The lateral lines represents the confidence interval.
Factors associated with UIA in stroke and TIA patients
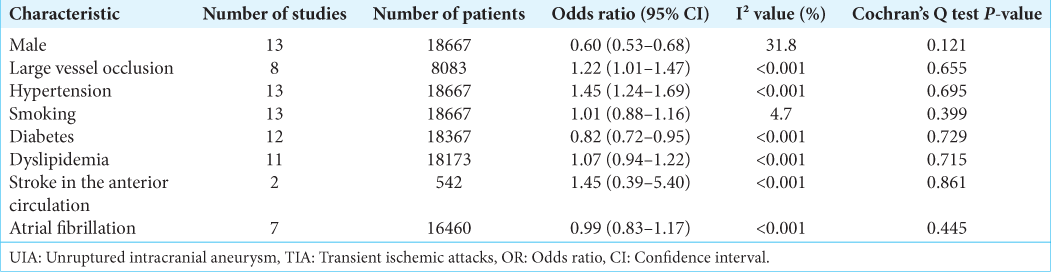
To identify the factors associated with UIA in stroke and TIA patients, we used a fixed-effects model and subsequently calculated pooled estimates for the following extracted factors: male sex, large vessel occlusion, hypertension, smoking, diabetes, dyslipidemia, stroke in the anterior circulation, and atrial fibrillation [
There was a statistically significant association between the presence of UIA in our study population and both large vessel occlusion (OR = 1.22, 95% CI = 1.01–1.47) and hypertension (OR = 1.45, 95% CI = 1.24–1.69). In contrast, male sex (OR = 0.60, 95% CI = 0.53–0.68) and diabetes (OR = 0.82, 95% CI = 0.72–0.95) demonstrated a significantly lower association with UIA presence, suggesting a protective role.
Smoking, dyslipidemia, atrial fibrillation, and anterior circulation stroke were not found to be significantly associated with UIA in stroke and TIA patients. Calculated I2 was <50%, confirmed by Cochrane Q tests that presented P-value >0.05 for all variables in our study, suggesting no substantial heterogeneity.
DISCUSSION
Intracranial aneurysms are not passively enlarging vascular structures, rather they are active, complex formations that exhibit features of inflammation, with pathophysiology similar to endothelial dysfunction found in vascular disease of stroke and TIA patients.[
In this meta-analysis, we determined the prevalence of UIA among ischemic stroke and TIA patients admitted to hospitals and identified factors associated with the development of UIA in this population by pooling data from 23 individual studies, totaling 25,420 stroke patients. We found the global prevalence rate to be 5% (95% CI = 4–6%), notably higher than the 2.8% reported in the general population.[
Our results revealed that factors such as female sex, hypertension, and large vessel occlusion were significantly associated with the presence of UIA in stroke and TIA patients. Prior studies suggest that the risk of intracranial aneurysms increases among females after menopause, especially those with early menopause.[
Similar to a previous study conducted by Vlak et al.,[
We also found that large vessel occlusion (OR = 1.22, 95% CI = 1.01–1.47) significantly correlated with UIA formation. We hypothesize that the high flow acceleration and turbulent hemodynamics may affect vessel walls in a similar mechanism to that of hypertension. These occlusions promote maladaptive inflammatory changes, remodeling, and repair pathways that ultimately create defective endothelial wall architecture, forming intracranial aneurysms.[
Patients with diabetes were found to have lower odds of developing intracranial aneurysms (OR = 0.82, 95% CI = 0.72–0.95). Paradoxically, this protective effect may be explained by the use of medications used to treat diabetes.[
Our work has important strengths and limitations. To date, this is one of the most thorough studies performed to evaluate UIA and their associated characteristics in stroke and TIA patients. The large number of patients and extensive search strategy contributed to the reliability of this study. In addition, our meta-analysis is the first to perform sensitivity analyses for the prevalence rate of UIA in stroke and TIA patients. The analyses revealed that excluding each of the studies from the pooled estimate had no influence on the prevalence rate across all studies globally, indicating the robustness of our findings. Nonetheless, the results of our primary outcome demonstrated considerable heterogeneity (I2 = 90.3%, P < 0.001) among all studies. To take this into consideration, we used the random-effects model to evenly redistribute the statistical weight of each study for analysis. Finally, data on the prevalence of UIA in stroke and TIA patients was limited to North America, Europe, East Asia, and Southeast Asia. Additional research should aim to assess UIA in stroke and TIA patients in South America, Africa, Oceania, and the rest of Asia for global applicability of these analyses.
CONCLUSION
Our meta-analysis suggests that pooled estimates of prevalence of UIA among patients with ischemic stroke and TIA admitted to hospitals is 5% worldwide, with a higher prevalence observed in North America and East Asia. Medical conditions and characteristics such as hypertension and large vessel occlusion were associated with increased risk of UIA in stroke and TIA patients, whereas diabetes and male sex were protective factors. However, the development of UIA in stroke and TIA patients had no association with smoking, dyslipidemia, atrial fibrillation, and stroke in the anterior circulation. Our findings provide helpful information for the recognition of UIA in high-risk populations to provide targeted and prompt control measures to prevent unfavored prognoses.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aoki T, Nishimura M. Targeting chronic inflammation in cerebral aneurysms: Focusing on NF-KB as a putative target of medical therapy. Expert Opin Ther Targets. 2010. 14: 265-73
2. Ballotta E, Giau GD, Manara R, Baracchini C. Extracranial severe carotid stenosis and incidental intracranial aneurysms. Ann Vasc Surg. 2006. 20: 5-8
3. Can A, Castro VM, Yu S, Dligach D, Finan S, Gainer VS. Antihyperglycemic agents are inversely associated with intracranial aneurysm rupture. Stroke. 2018. 49: 34-9
4. Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013. 44: 3613-22
5. Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH. Biology of intracranial aneurysms: Role of inflammation. J Cereb Blood Flow Metab. 2012. 32: 1659-76
6. Chalouhi N, Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF. Cigarette smoke and inflammation: Role in cerebral aneurysm formation and rupture. Mediators Inflamm. 2012. 2012: 271582
7. Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013. 44: 2594-7
8. Chen ML, Gupta A, Chatterjee A, Khazanova D, Dou E, Patel H. Association between unruptured intracranial aneurysms and downstream stroke. Stroke. 2018. 49: 2029-33
9. Chiu WT, Hong CT, Chi NF, Hu CJ, Hu HH, Chan L. The risk of intravenous thrombolysis-induced intracranial hemorrhage in Taiwanese patients with unruptured intracranial aneurysm. PLoS One. 2017. 12: e0180021
10. Ding C, Toll V, Ouyang B, Chen M. Younger age of menopause in women with cerebral aneurysms. J Neurointerv Surg. 2012. 5: 327-31
11. Doyle SJ, George BP, Holloway RG, Kelly AG. Incidental findings in radiographic imaging for in-patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2018. 27: 3131-6
12. Edwards NJ, Kamel H, Josephson SA. The safety of intravenous thrombolysis for ischemic stroke in patients with pre-existing cerebral aneurysms. Stroke. 2012. 43: 412-6
13. Goyal N, Tsivgoulis G, Zand R, Sharma VK, Barlinn K, Male S. Systemic thrombolysis in acute ischemic stroke patients with unruptured intracranial aneurysms. Neurology. 2015. 85: 1452-8
14. Guo J, Dhaliwall JK, Chan KK, Ghanim H, Al Koudsi N, Lam L. In vivo effect of insulin to decrease matrix metalloproteinase-2 and-9 activity after arterial injury. J Vasc Res. 2013. 50: 279-88
15. Héman LM, Jongen LM, Van der Worp HB, Rinkel GJ, Hendrikse J. Incidental intracranial aneurysms in patients with internal carotid artery stenosis. Stroke. 2009. 40: 1341-6
16. Herrera Ortiz AF, Camacho EC, Rojas JC, Camacho TC, Guevara SZ, Cuenca NT. A practical guide to perform a systematic literature review and meta-analysis. Princ Pract Clin Res J. 2021. 7: 47-57
17. Herrera Ortiz AF, Cuenca NT, Beaujon LJ. Brain changes in magnetic resonance imaging caused by child abuse: A systematic literature review. Rev Cuarzo. 2021. 27: 27-33
18. Hokari M, Isobe M, Imai T, Chiba Y, Iwamoto N, Isu T. The impact of atherosclerotic factors on cerebral aneurysm is location dependent: Aneurysms in stroke patients and healthy controls. J Stroke Cerebrovasc Dis. 2014. 23: 2301-7
19. Hurford R, Taveira I, Kuker W, Rothwell PM. Prevalence, predictors and prognosis of incidental intracranial aneurysms in patients with suspected TIA and minor stroke: A population-based study and systematic review. J Neurol Neurosurg Psychiatry. 2020. 92: 542-8
20. Ikawa F, Morita A, Tominari S, Nakayama T, Shiokawa Y, Date I. Rupture risk of small unruptured cerebral aneurysms. J Neurosurg. 2020. 132: 69-78
21. Ishikawa Y, Hirayama T, Nakamura Y, Ikeda K. Incidental cerebral aneurysms in acute stroke patients: Comparison of asymptomatic healthy controls. J Neurol Sci. 2010. 298: 42-5
22. Jamous MA, Nagahiro S, Kitazato KT, Tamura T, Kuwayama K, Satoh K. Role of estrogen deficiency in the formation and progression of cerebral aneurysms. Part II: experimental study of the effects of hormone replacement therapy in rats. J Neurosurg. 2005. 103: 1052-7
23. Jiranukool J, Thiarawat P, Galassi W. Prevalence of intracranial aneurysms among acute ischemic stroke patients. Surg Neurol Int. 2020. 11: 341
24. Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms. Stroke. 2001. 32: 485-91
25. Kanesa-Thasan R, Cox M, Patel M, Curtis B, Hurst RW, Kung D. Actionable vascular and other incidental findings on CTA in patients undergoing acute stroke intervention. Neuroradiol J. 2018. 31: 572-7
26. Kappelle L, Eliasziw M, Fox A, Barnett HM. Small, unruptured intracranial aneurysms and management of symptomatic carotid artery stenosis. Neurology. 2000. 55: 307-9
27. Kim JH, Suh SH, Chung J, Oh YJ, Ahn SJ, Lee KY. Prevalence and characteristics of unruptured cerebral aneurysms in ischemic stroke patients. J Stroke. 2016. 18: 321-7
28. Kim JT, Park MS, Yoon W, Cho KH. Detection and significance of incidental unruptured cerebral aneurysms in patients undergoing intravenous thrombolysis for acute ischemic stroke. J Neuroimaging. 2012. 22: 197-200
29. Kim SC, Singh M, Huang J, Prestigiacomo CJ, Winfree CJ, Solomon RA. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997. 41: 642-7
30. Lai LT, Morgan MK, Patel NJ. Smoking increases the risk of de novo intracranial aneurysms. World Neurosurg. 2014. 82: e195-201
31. Lindbohm JV, Kaprio J, Jousilahti P, Salomaa V, Korja M. Risk factors of sudden death from subarachnoid hemorrhage. Stroke. 2017. 48: 2399-404
32. Mackey J, Khoury JC, Alwell K, Moomaw CJ, Kissela BM, Flaherty ML. Stable incidence but declining case-fatality rates of subarachnoid hemorrhage in a population. Neurology. 2016. 87: 2192-7
33. Melsen W, Bootsma M, Rovers M, Bonten M. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014. 20: 123-9
34. Metaxa E, Tremmel M, Natarajan SK, Xiang J, Paluch RA, Mandelbaum M. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke. 2010. 41: 1774-82
35. Mittal MK, Seet RC, Zhang Y, Brown RD, Rabinstein AA. Safety of intravenous thrombolysis in acute ischemic stroke patients with saccular intracranial aneurysms. J Stroke Cerebrovasc Dis. 2013. 22: 639-43
36. Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage. Stroke. 2001. 32: 606-12
37. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009. 339: b2535
38. Mowla A, Singh K, Mehla S, Ahmed MK, Shirani P, Kamal H. Is acute reperfusion therapy safe in acute ischemic stroke patients who harbor unruptured intracranial aneurysm?. Int J Stroke. 2015. 10: 113-8
39. Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992. 7: 120-5
40. Oh YS, Shon YM, Kim BS, Cho AH. Long-term follow-up of incidental intracranial aneurysms in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013. 22: 329-33
41. Oh YS, Lee SJ, Shon YM, Yang DW, Kim BS, Cho AH. Incidental unruptured intracranial aneurysms in patients with acute ischemic stroke. Cerebrovasc Dis. 2008. 26: 650-3
42. Patel K, Zafar M, Ziganshin B, Elefteriades J. Diabetes mellitus: Is it protective against aneurysm? A narrative review. Cardiology. 2018. 141: 107-22
43. Reggiani F, Labanca V, Mancuso P, Rabascio C, Talarico G, Orecchioni S. Adipose progenitor cell secretion of GMCSF and MMP9 promotes a stromal and immunological microenvironment that supports breast cancer progression. Cancer Res. 2017. 77: 5169-82
44. Rong Z, Li L, Fei F, Luo L, Qu Y. Combined treatment of glibenclamide and CoCl2 decreases MMP9 expression and inhibits growth in highly metastatic breast cancer. J Exp Clin Cancer Res. 2013. 32: 32
45. Sheth KN, Shah N, Morovati T, Hermann LD, Cronin CA. Intravenous rt-PA is not associated with increased risk of hemorrhage in patients with intracranial aneurysms. Neurocrit Care. 2012. 17: 199-203
46. Shono Y, Sugimori H, Matsuo R, Fukushima Y, Wakisaka Y, Kuroda J. Safety of antithrombotic therapy for patients with acute ischemic stroke harboring unruptured intracranial aneurysm. Int J Stroke. 2018. 13: 734-42
47. Starke RM, Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr Neurovasc Res. 2013. 10: 247-55
48. Texakalidis P, Sweid A, Mouchtouris N, Peterson EC, Sioka C, Rangel-Castilla L. Aneurysm formation, growth, and rupture: The biology and physics of cerebral aneurysms. World Neurosurg. 2019. 130: 277-84
49. Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES. Guidelines for the management of patients with unruptured intracranial aneurysms. Stroke. 2015. 46: 2368-400
50. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36
51. Vlak MH, Rinkel GJ, Greebe P, Algra A. Independent risk factors for intracranial aneurysms and their joint effect. Stroke. 2013. 44: 984-7
52. Wiebers DO. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10
53. Wu X, Duan Z, Liu Y, Zhou C, Jiao Z, Zhao Y. Incidental unruptured intracranial aneurysms do not impact outcome in patients with acute cerebral infarction. Front Neurol. 2021. 12: 613027
54. Zibold F, Kleine JF, Zimmer C, Poppert H, Boeckh-Behrens T. Aneurysms in the target vessels of stroke patients subjected to mechanical thrombectomy: Prevalence and impact on treatment. J NeuroInterv Surg. 2015. 8: 1016-20
55. Zlowodzki M, Poolman RW, Kerkhoffs GM, Tornetta P, Bhandari M. How to interpret a meta-analysis and judge its value as a guide for clinical practice. Acta Orthop. 2007. 78: 598-609