- Department of Neuroscience, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia.
- Department of Radiology, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia.
- Department of Pathology, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia.
Correspondence Address:
Mosab Abbas, Department of Neuroscience, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia.
DOI:10.25259/SNI_929_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mosab Abbas1, Mariam Zuhair Enani1, Zehour Alsabban2, Abdelrazak Meliti3, Mohammed Homoud1. Primary anterior visual pathway germinoma in a 13-year-old boy: A case report. 16-Feb-2024;15:48
How to cite this URL: Mosab Abbas1, Mariam Zuhair Enani1, Zehour Alsabban2, Abdelrazak Meliti3, Mohammed Homoud1. Primary anterior visual pathway germinoma in a 13-year-old boy: A case report. 16-Feb-2024;15:48. Available from: https://surgicalneurologyint.com/surgicalint-articles/12754/
Abstract
Background: Primary optic nerve and chiasmal germinomas are very rare. These lesions can commonly be mistaken for optic pathway gliomas based on imaging alone. It is radiosensitive and cured in most of the cases.
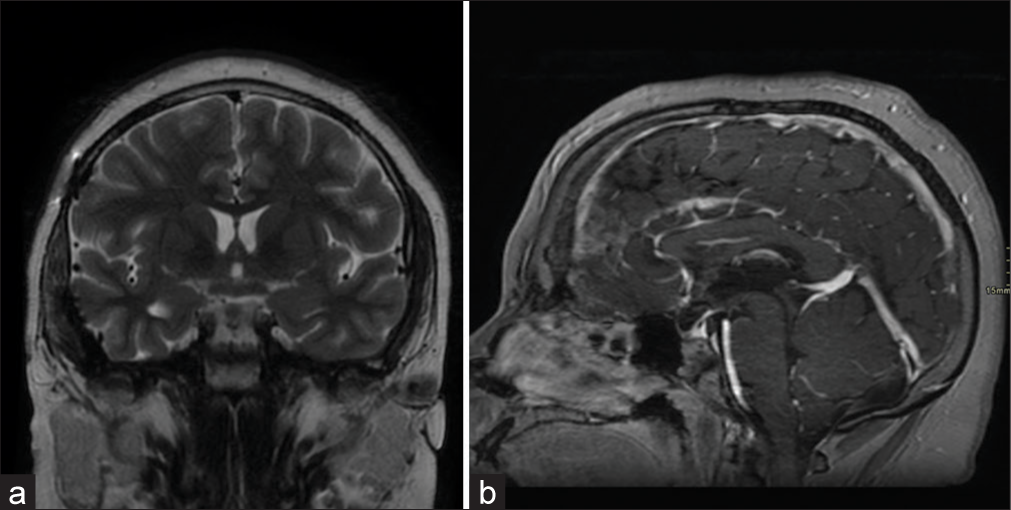
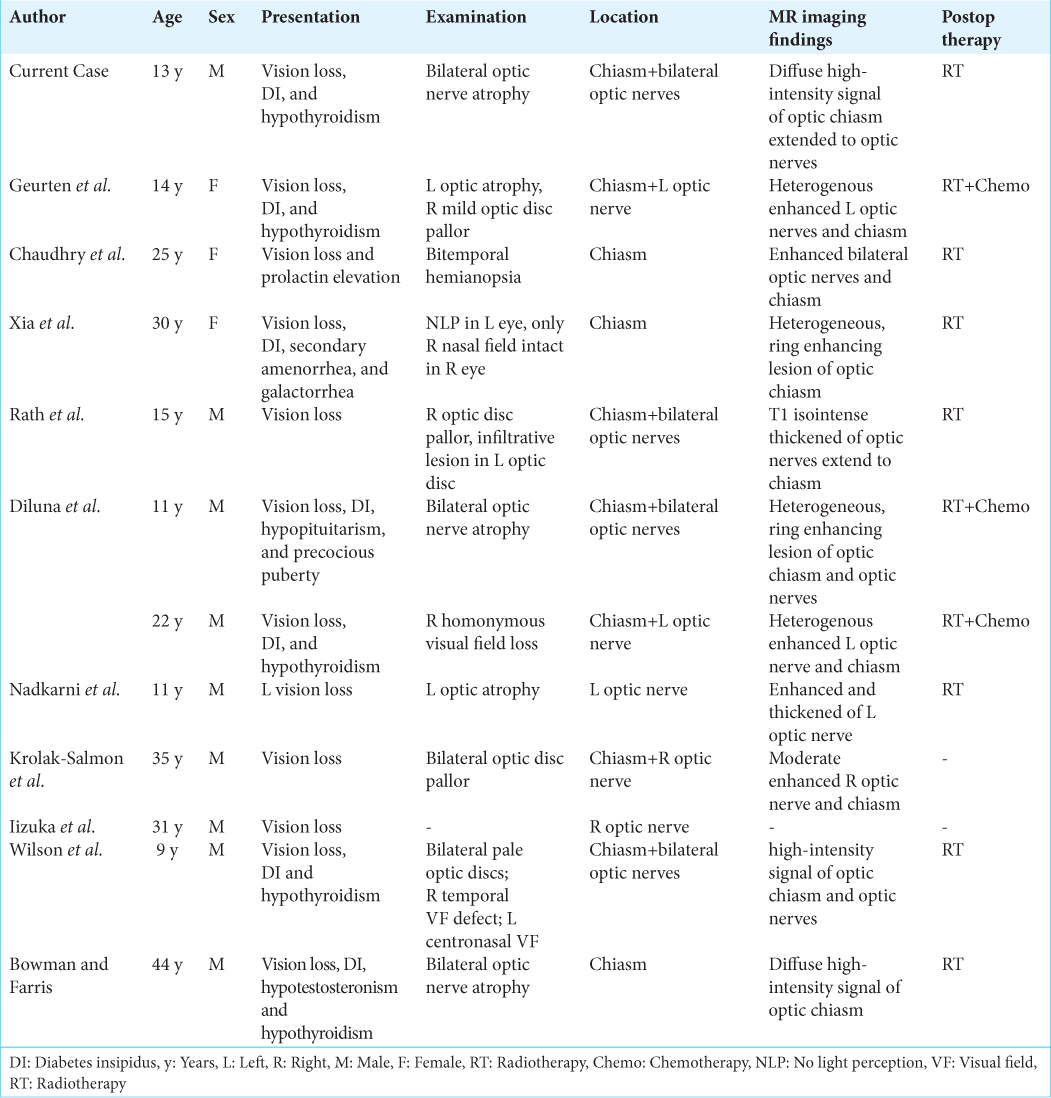
Case Description: We report a rare case of a 13-year-old boy with primary bilateral optic nerves and chiasmal germinoma who underwent partial surgical resection followed by radiotherapy. Follow-up brain imaging after two months post-radiotherapy showed interval regression of the tumor. Our literature review identified that 12 reported cases of primary anterior visual pathway germinoma had been reported to regress significantly post-radiotherapy alone or with chemotherapy.
Conclusion: Histologic correlation is essential for appropriate treatment, alleviating symptoms, and avoiding irreversible vision loss.
Keywords: Chiasmal germinoma, Germ cell tumors, Intracranial germinoma, Optic nerves germinoma
INTRODUCTION
Intracranial germ cell tumors (GCTs) comprise <1% of all central nervous system (CNS) tumors[
CASE DESCRIPTION
A 13-year-old boy who was born to second-degree consanguineous parents was not known to have any medical illness. He presented to the emergency department of King Faisal Specialist Hospital and Research Center at Jeddah with a persistent headache for four weeks, followed by progressive visual loss for over two weeks associated with polyurea before the presentation. The parents denied having a similar condition or history of any malignancies in the family. On examination, the pupils were dilated bilaterally with a sluggish reaction in the left eye. The ophthalmologic assessment revealed diminished visual acuity with no light perception in the right eye and light perception OS. A fundoscopic examination showed bilateral optic atrophy. No skin stigmata or caféau-lait spots were inspected. A brain magnetic resonance imaging (MRI) revealed a lobulated enhancing lesion arising primarily from the optic chiasm involving the prechiasmatic optic nerves and the hypothalamus, measuring 3.1 × 3.7 × 1.7 cm (anteroposterior × mediolateral × craniocaudal dimensions).
The infundibulum was normal. Radiologic findings were most suggestive of optic pathway glioma [
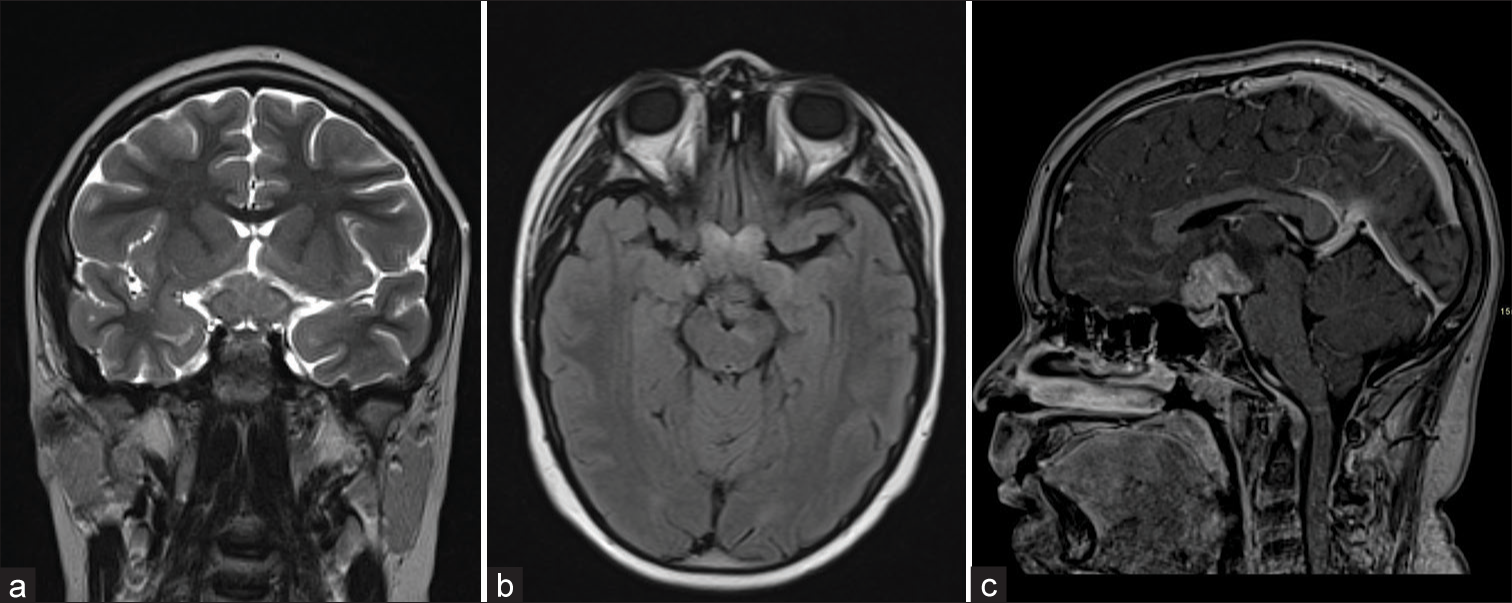
Figure 1:
(a) Coronal T2-weighted image shows lobulated thickening of the optic chiasm with normal pituitary infundibulum and (b) axial fluid-attenuated inversion recovery shows the involvement of pre-chiasmatic optic nerves and optic tracts, (c) midsagittal contrast-enhanced T1-weighted spoiled gradient recalled echo demonstrates the avid lesion enhancement with involvement of the hypothalamus.
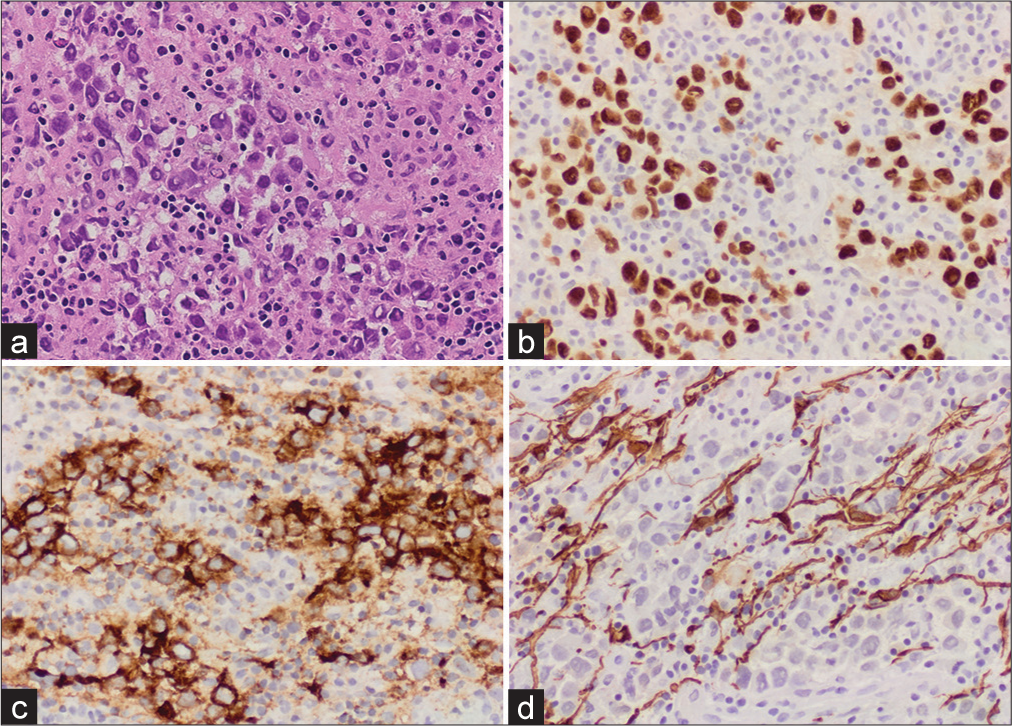
Figure 2:
(a) Hematoxylin and eosin stained section (H&E @ ×20), showing nests of slightly discohesive, large epithelioid cells with abundant cytoplasm and pleomorphic nuclei intermixed with benign lymphocytes, (b) immunohistochemistry reveals positive immunoreactivity against SALL4, OCT4, (c) immunohistochemistry reveals positive immunoreactivity against placental alkaline phosphatase (PLAP), and (d) negative immunohistochemistry for glial fibrillary acidic protein (GFAP), S100, AE1/AE3, CD45, and CD30.
DISCUSSION
GCTs classified by the 2021 World Health Organization classification of the tumors of the CNS into mature teratoma, immature teratoma, teratoma with somatic-type malignancy, germinoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and mixed GCT.[
CONCLUSION
Primary intracranial optic and chiasmal germinoma should be considered in the differential diagnosis of optic pathway lesions. Brain imaging alone can be suboptimal in differentiating between anterior visual pathway germinoma and gliomas. Histologic correlation is essential for appropriate treatment, alleviating symptoms, and avoiding irreversible vision loss.
Ethical approval
King Faisal Specialist Hospital and Research Center Institutional review board approved the case report. Study Number: IRB 2020-CR-25.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al-Hussaini M, Sultan I, Abuirmileh N, Jaradat I, Qaddoumi I. Pineal gland tumors: Experience from the SEER database. J Neurooncol. 2009. 94: 351-8
2. Bowman CB, Farris BK. Primary chiasmal germinoma. A case report and review of the literature. J Clin Neuroophthalmol. 1990. 10: 9-17
3. Chaudhry NS, Ahmad FU, Whittington E, Schatz N, Morcos JJ. Primary intrinsic chiasmal germinoma. J Neuroophthalmol. 2015. 35: 171-4
4. Diluna ML, Two AM, Levy GH, Patel T, Huttner AJ, Duncan CC. Primary, non-exophytic, optic nerve germ cell tumors. J Neurooncol. 2009. 95: 437-43
5. Erkal HŞ, Serin M, Çakmak A. Management of optic pathway and chiasmatic-hypothalamic gliomas in children with radiation therapy. Radiother Oncol. 1997. 45: 11-5
6. Geurten C, Pavon-Mengual M, Lo WB, Bowen C, Gagen R, Stirling H. Optic nerve germinoma and transient spontaneous regression-more than meets the eye. J Pediatr Hematol Oncol. 2022. 44: 255-60
7. Hoei-Hansen CE, Sehested A, Juhler M, Lau YF, Skakkebaek NE, Laursen H. New evidence for the origin of intracranial germ cell tumours from primordial germ cells: Expression of pluripotency and cell differentiation markers. J Pathol. 2006. 209: 25-33
8. Hoffman HJ, Otsubo H, Hendrick EB, Humphreys RP, Drake JM, Becker LE. Intracranial germ-cell tumors in children. J Neurosurg. 1991. 74: 545-51
9. Iizuka H, Nojima T, Kadoya S. Germinoma of the optic nerve: Case report. Noshuyo Byori. 1996. 13: 95-8
10. Jaing TH, Lin KL, Tsay PK, Hsueh C, Hung PC, Wu CT. Treatment of optic pathway hypothalamic gliomas in childhood: Experience with 18 consecutive cases. J Pediatr Hematol Oncol. 2008. 30: 222-4
11. Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: Natural history and pathogenesis. J Neurosurg. 1985. 63: 155-67
12. Krolak-Salmon P, Androdias G, Honnorat J, Caudie C, Bret P, Vighetto A. Case report Beware of optic neuritis ! Case report. Lancet. 2002. 1: 516-7
13. Kyritsis AP. Management of primary intracranial germ cell tumors. J Neurooncol. 2010. 96: 143-9
14. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
15. Madden J, Foreman NK, Liu AK. Germ cell tumors of the brainstem: Report on two cases with pulmonary complications and a review of the literature. J Neurooncol. 2009. 93: 405-8
16. Nadkarni TD, Fattepurkar SC, Desai KI, Goel A. Intracranial optic nerve germinoma. J Clin Neurosci. 2004. 11: 559-61
17. Nakase H, Ohnishi H, Touho H, Karasawa J, Tsunoda S. Cerebellar primary germ-cell tumor in a young boy. Brain Dev. 1994. 16: 396-8
18. Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017. 19: v1-88
19. Panyaping T, Taebunpakul P, Tritanon O. Accuracy of apparent diffusion coefficient values and magnetic resonance imaging in differentiating suprasellar germinomas from chiasmatic/hypothalamic gliomas. Neuroradiol J. 2020. 33: 201-9
20. Rath S, Vemuganti GK, Biswas G, Mod H. Optic nerve and chiasmal germinoma. Ophthal Plast Reconstr Surg. 2009. 25: 161-3
21. Wilson JT, Wald SL, Aitken PA, Mastromateo J, Vieco PT. Primary diffuse chiasmatic germinomas: Differentiation from optic chiasm gliomas. Pediatr Neurosurg. 1995. 23: 1-6
22. Xia C, Liu Z, Zhang R, Mao Y, Wang Y. Primary intrinsic optic chiasm germinoma. J Clin Neurosci. 2011. 18: 860-2