- Department of Neurosurgery, Arturo Montiel Rojas Medical Center, Instituto de Seguridad Social del Estado de México y Municipios, Metepec, Mexico
- Department of Pathology, Dr José Luis Barrera Franco, State Oncology Center, Toluca, Mexico.
Correspondence Address:
Elizabeth Escamilla Chávez, Department of Neurosurgery, Arturo Montiel Rojas Medical Center, Instituto de Seguridad Social del Estado de México y Municipios, Metepec, México.
DOI:10.25259/SNI_65_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Elizabeth Escamilla Chávez1, Julio César Delgado Arce1, Edinson David Berrio Perea1, Abraham Gallegos Pedraza1, Ana Itiel Jimenez Ávila1, David Eduardo Aguirre Quezada2, Pablo David Guerrero Suárez1. Primary central nervous system lymphoma: A mirror type presentation in an immunocompetent patient. 26-Apr-2024;15:143
How to cite this URL: Elizabeth Escamilla Chávez1, Julio César Delgado Arce1, Edinson David Berrio Perea1, Abraham Gallegos Pedraza1, Ana Itiel Jimenez Ávila1, David Eduardo Aguirre Quezada2, Pablo David Guerrero Suárez1. Primary central nervous system lymphoma: A mirror type presentation in an immunocompetent patient. 26-Apr-2024;15:143. Available from: https://surgicalneurologyint.com/surgicalint-articles/12874/
Abstract
Background: Primary central nervous system (CNS) lymphoma is a very rare extranodal non-Hodgkin lymphoma. The bilateral pattern, as we call it “mirror type”, has been identified in other CNS lesions such as gliomas, metastases, and demyelinating lesions, so the differential diagnosis includes imaging studies such as magnetic resonance imaging contrasted with spectroscopy, ruling out immunodeficiency or metastatic disease.
Case Description: A 65-year-old female presented progressing headache, loss of memory and language alterations, as well as sensory alterations. Neuroimaging showed the presence of two equidistant periventricular lesions at the level of both ventricular atria, a spectroscopy study suggestive of malignancy. Serological studies showed no evidence of immunodeficiency or the presence of positive tumor markers; however, a biopsy was performed, which revealed a histopathological result of primary lymphoma of the CNS.
Conclusion: In neuro-oncology, primary CNS tumors with multiple lesions are rare, even more, the “mirror type” lesions. Lymphomas are lesions that can present in different ways on imaging and clinical presentation. These tumors that present a vector effect due to their size, perilesional edema, or that lead to loss of neurological function are highly discussed in diagnostic and surgical treatment. Due to their prognosis, action on diagnosis and treatment must be taken as quickly as hospital resources allow.
Keywords: Central nervous system-diffuse large B-cell lymphoma (CNS-DLBL), Mirror type tumor, Primary central nervous system lymphoma (PCNSL), Primary lymphoma
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is a very rare extranodal non-Hodgkin lymphoma that accounts for 2.4–3% of all primary central nervous system (CNS) tumors and 4–6% of all extranodal lymphomas.[
Due to the poor prognosis of PCNSL, management protocols with biopsy without prior use of steroids, chemotherapy with methotrexate, and sometimes immunotherapy with anti-CD20 rituximab or radiotherapy are the indicated standard.[
CASE DESCRIPTION
A 65-year-old female with a history of type 2 diabetes mellitus, systemic arterial hypertension and long-standing right hearing loss. She began his condition 21 days before his initial evaluation in July 2023, presenting with moderate to intense holocranial headache that partially subsided with the use of non-steroidal anti-inflammatory drugs. She had previously been diagnosed with dementia due to retrograde memory and language disorders. In the neurological examination with Glasgow 13 points (O:4, V:3, M:6), mental functions with global aphasia, isocoria of 3 mm, preserving photomotor and consensual reflexes, right side conduction hearing loss, preserved motor function, sensitive without response to pain in the right hemibody. No evidence of intracranial hypertension or history of seizures. A simple and contrasted tomography was performed, showing the presence of two lesions at the level of both ventricular trigone each one apparently, which were confirmed by magnetic resonance imaging (MRI) scan indicating periventricular lesions with extension to the white matter of the temporal and parietal lobes, a clear reduction of the ventricular space of both atrium was observed, no communication between these lesions through fibers of the corpus callosum, with mostly uniform enhancement at gadolinium contrast [
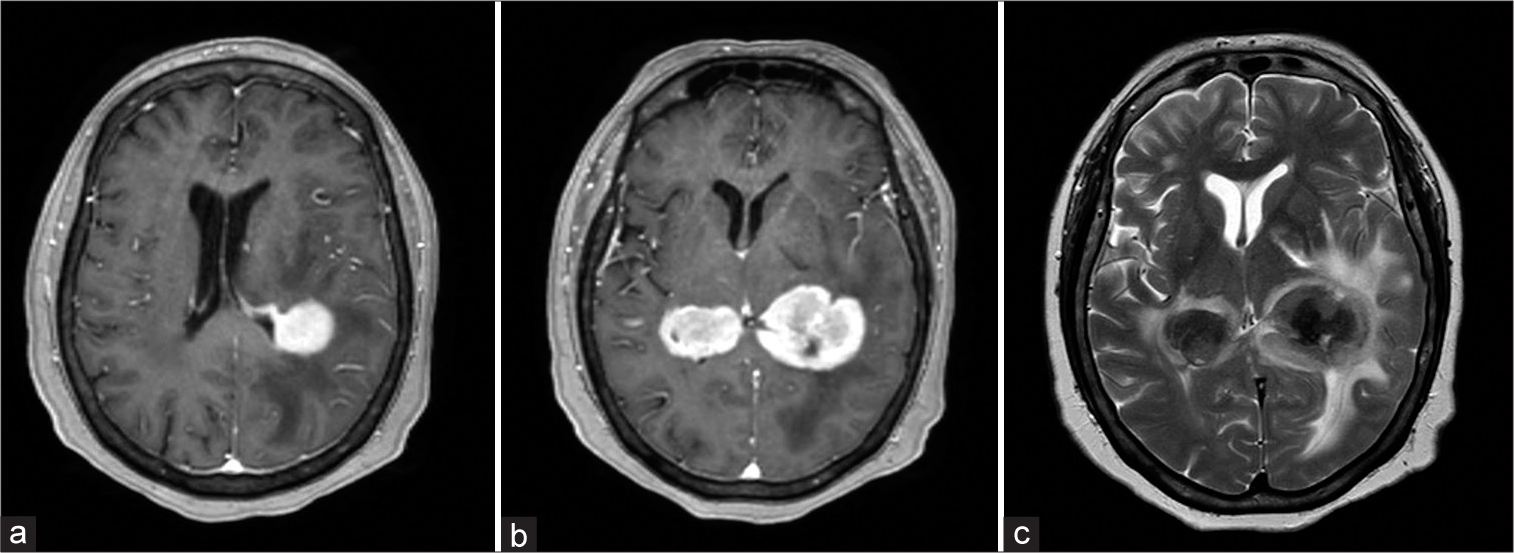
Figure 1:
(a) T1-weighted axial-enhanced magnetic resonance imaging sequence, a periventricular lesion is observed on the left lateral ventricle with extension to the white matter of the parietal lobe, the right lateral ventricle is observed without alterations. (b) Two well-defined lesions are observed at the level of the ventricular atrium, and a slightly heterogeneous center is observed. (c) Moderate perilesional edema was observed on the T2 sequence with predominance in the left lesion and with an evident hypointense center. No communication of both lesions through the corpus callosum was identified.
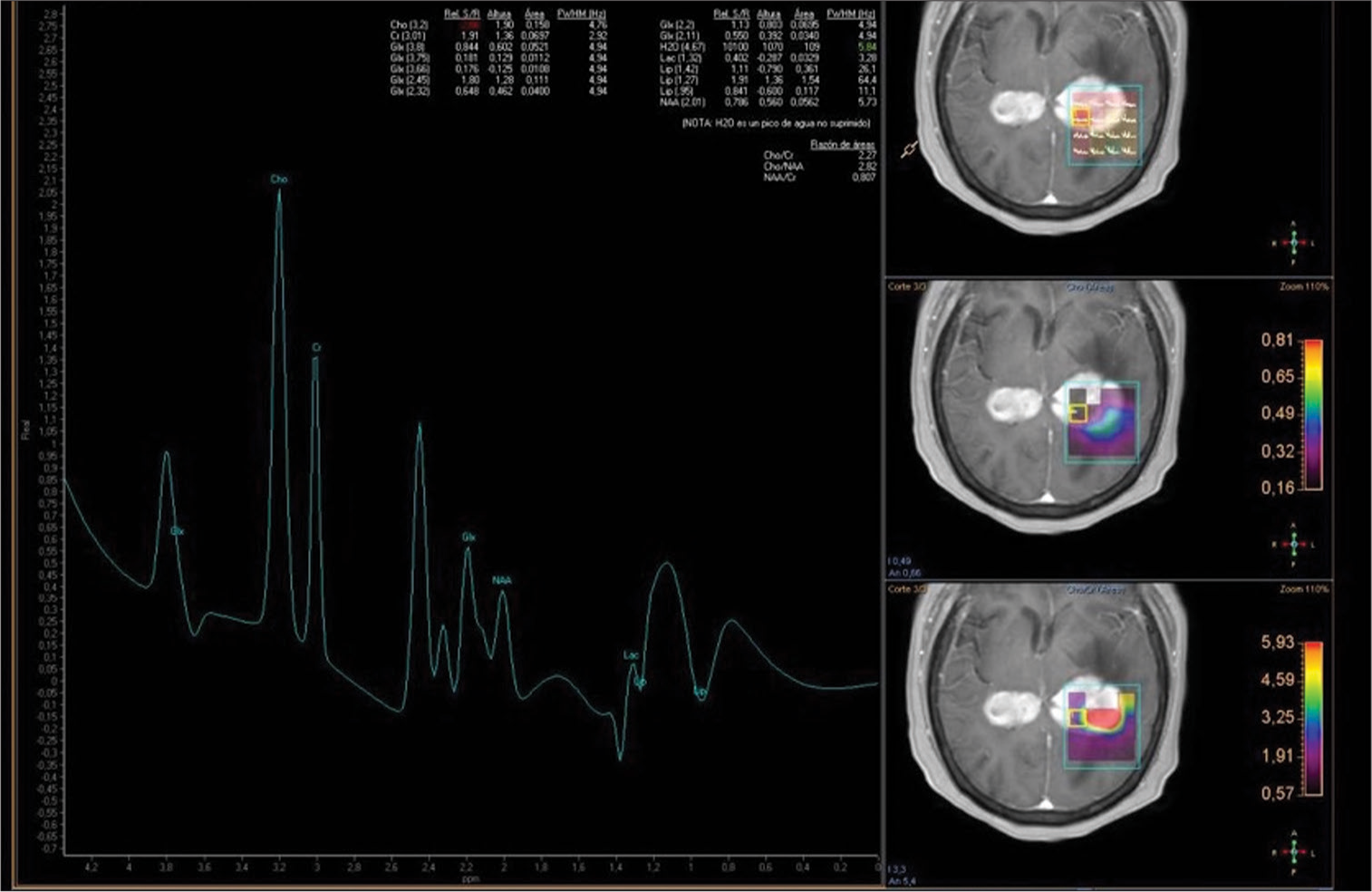
Figure 2:
A T1-weighted gadolinium-enhanced magnetic resonance imaging spectroscopy with voxel within the enhancement center of the lesion, at the level of the left ventricular atrium, with the signal of an elevated choline peak, as well as creatine and a reduced N-acetyl aspartate peak. There is an elevated choline/creatine ratio of 2.27 and elevated lipids/lactate consistent with areas of necrosis.
A temporal craniotomy with a transulcal T2–T3 approach guided by a neuronavigation system was performed. Biopsy samples were taken. The tumor was identified as infiltrative and diffused in the white matter, with gray color, soft consistency, and necrotic core. The ventricle was not opened. No complications were presented.
Histopathology revealed diffuse large B-cell lymphoma with an angiocentric pattern with positive immunohistochemical staining for CD20 and Ki67 in 70% [
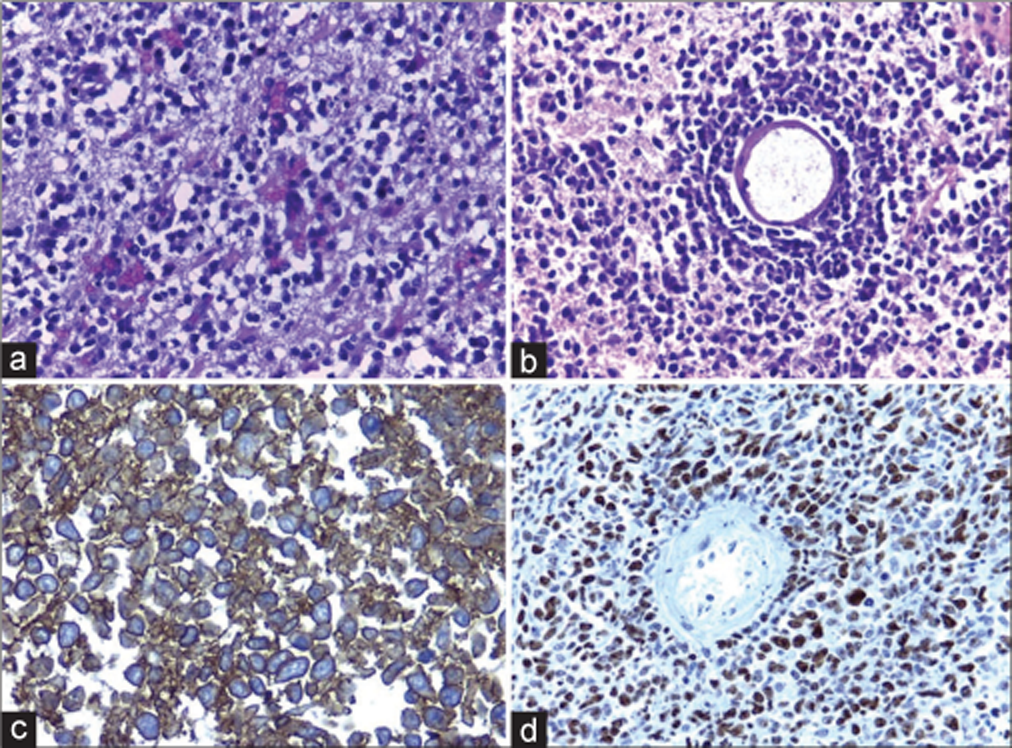
Figure 3:
(a) Diffuse proliferation of mature lymphocytes infiltrating the brain tissue is observed. (b) Typical angiocentric arrangement. (c) Intense positive immunoreaction for CD20 in the membrane and cytoplasm. (d) Positive immunochemical expression for Ki-67:70%, related to a high proliferation index.
The patient had a complicated course due to pneumonia, was discharged from the hospital 15 days after surgery, and was referred to an oncology center.
DISCUSSION
The latest World Health Organization 2021 update classifies the main CNS lymphomas as primary diffuse large B-cell lymphoma of the CNS (CNS-DLBL), immunodeficiency-associated CNS lymphomas, lymphomatoid granulomatosis, and intravascular large B-cell lymphoma. CNS-DLBL is solitary lesions in 65% of cases, located in the cerebral hemispheres 38%, thalami and basal nuclei 16%, corpus callosum 14%, periventricular 12%, or cerebellum 9%.[
High-grade glioma is a malignant infiltrative lesion of the white matter that may have image similarities to the case presented, such as multicentric glioma, which represents <2% of patients with malignant gliomas, are periventricular and does not involve the corpus callosum.[
PCNSL are characteristically iso-hyperdense on non-contrast tomography, with hypointense iso-a enhancement on T2. In immunocompetent patients, when contrast is passed, a marked and homogeneous enhancement is observed due to the alteration of the blood-brain barrier; hemorrhage, calcification, necrosis, and cyst formation are unusual.[
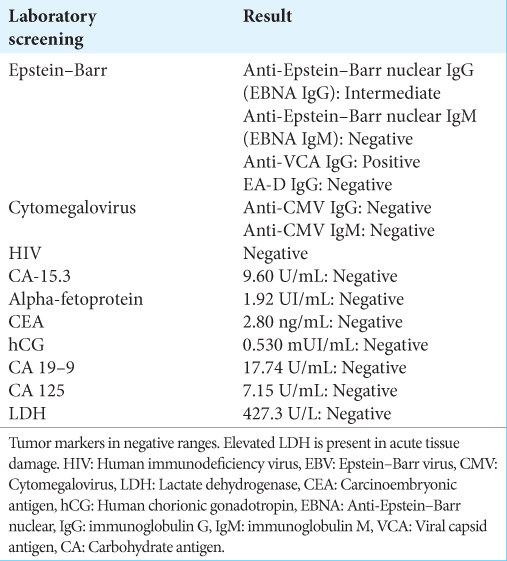
Regarding the viral panel for EBV, the values of anti-Epstein– Barr nuclear immunoglobulin G (IgG) were observed as intermediate values according to our laboratory parameters, as well as anti-Epstein–Barr viral capsid antigen IgG (anti- VCA IgG) with positive values, which indicate a history of infection, ruling out active infection [
Current international guidelines[
CONCLUSION
In neuro-oncology, primary CNS tumors with multiple lesions are rare, even more, the “mirror type” lesions. Lymphomas are lesions that can present in different ways on imaging and clinical presentation. These tumors that present a vector effect due to their size, perilesional edema or that lead to loss of neurological function are highly discussed in the diagnostic and surgical treatment. Due to their prognosis, action on diagnosis and treatment must be taken as quickly as hospital resources allow.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdel Razek AA, El-Serougy L, Abdelsalam M, Gaballa G, Talaat M. Differentiation of primary central nervous system lymphoma from glioblastoma: Quantitative analysis using arterial spin labeling and diffusion tensor imaging. World Neurosurg. 2019. 123: e303-9
2. Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M, Guy G, Lapierre F. Primary intracerebral malignant lymphoma: Report of 248 cases. J Neurosurg. 2000. 92: 261-6
3. Bullis CL, Maldonado-Perez A, Bowden SG, Yaghi N, Munger D, Wood MD. Diagnostic impact of preoperative corticosteroids in primary central nervous system lymphoma. J Clin Neurosci. 2020. 72: 287-91
4. Chen T, Liu Y, Wang Y, Chang Q, Wu J, Wang Z. Evidence-based expert consensus on the management of primary central nervous system lymphoma in China. J Hematol Oncol. 2022. 15: 136
5. Chojak R, Koźba-Gosztyła M, Polańska K, Rojek M, Chojko A, Bogacz R. Surgical resection versus biopsy in the treatment of primary central nervous system lymphoma: A systematic review and meta-analysis. J Neurooncol. 2022. 160: 753-61
6. Da Silva AJ, Castro Pinheiro Gomes FE, Barros Pimentel Tenório GM, Pinto Almeida LM. Diffuse large B-cell lymphoma in bilateral basal ganglia: A rare case report. Cureus. 2022. 14: e32743
7. Dandachi D, Ostrom QT, Chong I, Serpa JA, Giordano TP, Kruchko C. Primary central nervous system lymphoma in patients with and without HIV infection: A multicenter study and comparison with U.S national data. Cancer Causes Control. 2019. 30: 477-88
8. De Wilde V, Dierickx D, Schroyens W, Van E, Neste D, Bonnet C. BHS guidelines for primary central nervous system lymphoma. Belg J Hematol. 2016. 7: 69-78
9. Gómez Roselló E, Quiles Granado AM, Laguillo Sala G, Pedraza Gutiérrez S. Primary central nervous system lymphoma in immunocompetent patients: Spectrum of findings and differential characteristics. Radiologia ((Engl Ed). 2018. 60: 280-9
10. Grommes C, Deangelis LM. Primary CNS lymphoma. J Clin Oncol. 2017. 35: 2410-8
11. Hajtovic S, Liu C, Diefenbach CM, Placantonakis DG. Epstein-barr virus-positive primary central nervous system lymphoma in a 40-year-old immune-competent patient. Cureus. 2021. 13: e12754
12. Hoang-Xuan K, Deckert M, Ferreri AJ, Furtner J, Gallego Perez-Larraya J, Henriksson R. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro Oncol. 2023. 25: 37-53
13. Inoue A, Matsumoto S, Ohnishi T, Miyazaki Y, Kinnami S, Kanno K. What is the best preoperative quantitative indicator to differentiate primary central nervous system lymphoma from glioblastoma?. World Neurosurg. 2023. 172: e517-23
14. Kaulen LD, Galluzzo D, Hui P, Barbiero F, Karschnia P, Huttner A. Prognostic markers for immunodeficiency-associated primary central nervous system lymphoma. J Neurooncol. 2019. 144: 107-15
15. Kayed MH, Saleh TR, Reda IS, Elsirafy MN, Farhoud AH, Abdelzaher E. The added value of advanced neuro-imaging (MR diffusion, perfusion and proton spectroscopy) in diagnosis of primary CNS lymphoma. Alexandria J Med. 2014. 50: 303-10
16. Kerr JR. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol. 2019. 72: 651-8
17. Kwok HM, Li KY, Chan RL, Chan CH, Hon Wong SK, Lee CM. Different facets of intracranial central nervous system lymphoma and its imaging mimics. J Clin Imaging Sci. 2022. 12: 4
18. Labak CM, Holdhoff M, Bettegowda C, Gallia GL, Lim M, Weingart JD. Surgical resection for primary central nervous system lymphoma: A systematic review. World Neurosurg. 2019. 126: e1436-48
19. Layden BT, Dubner S, Toft DJ, Kopp P, Grimm S, Molitch ME. Primary CNS lymphoma with bilateral symmetric hypothalamic lesions presenting with panhypopituitarism and diabetes insipidus. Pituitary. 2011. 14: 194-7
20. Li Y, Zhang ZX, Huang GH, Xiang Y, Yang L, Pei YC. A systematic review of multifocal and multicentric glioblastoma. J Clin Neurosci. 2021. 83: 71-6
21. Lu JQ, O’Kelly C, Girgis S, Emery D, Power C, Blevins G. Neuroinflammation preceding and accompanying primary central nervous system lymphoma: Case Study and literature review. World Neurosurg. 2016. 88: 692.e1-8
22. Mendez JS, Ostrom QT, Gittleman H, Kruchko C, Deangelis LM, Barnholtz-Sloan JS. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol. 2018. 20: 687-94
23. Morales-Martinez A, Lozano-Sanchez F, Duran-Peña A, Hoang-Xuan K, Houillier C. Primary central nervous system lymphoma in elderly patients: Management and perspectives. Cancers (Basel). 2021. 13: 3479
24. Nakagawa T, Arita H, Kijima N, Fujita J, Nagate Y, Hirayama R. Primary central nervous system lymphoma of the bilateral Bochdalek’s flower baskets: A case report. Interdiscip Neurosurg. 2020. 21: 100756
25. Norden AD, Drappatz J, Wen PY, Claus EB. Survival among patients with primary central nervous system lymphoma, 1973-2004. J Neurooncol. 2011. 101: 487-93
26. Norrington M, Rathi N, Jenkinson MD, Mills SJ. Neuroinflammation preceding primary central nervous system lymphoma (PCNSL)-case reports and literature review. J Clin Neurosci. 2021. 89: 381-8
27. Rae AI, Mehta A, Cloney M, Kinslow CJ, Wang TJ, Bhagat G. Craniotomy and survival for primary central nervous system lymphoma. Clin Neurosurg. 2019. 84: 935-44
28. Singhal S, Antoniou E, Kwan E, Gregory G, Lai LT. Cytoreductive surgery for primary central nervous system lymphoma: Is it time to consider extent of resection?. J Clin Neurosci. 2022. 106: 110-6
29. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011. 105: 1414-8
30. Wang P, Shi YH, Li JY, Zhang CZ. Differentiating glioblastoma from primary central nervous system lymphoma: The value of shaping and nonenhancing peritumoral hyperintense gyral lesion on FLAIR imaging. World Neurosurg. 2021. 149: e696-704
31. WHO Classification of Tumors Editorial Bor, editors. Central nervous system tumours. Lyon, France: International Agency for Research on Cancer; 2021. p. 6
32. Xiao DD, Yan PF, Wang YX, Osman MS, Zhao HY. Glioblastoma and primary central nervous system lymphoma: Preoperative differentiation by using MRI-based 3D texture analysis. Clin Neurol Neurosurg. 2018. 173: 84-90