- Department of Neurological Surgery, University of Miami Hospital, Miami, Florida, United States,
- Department of Neurosurgery, Affiliated Hospital of Atitus Education School of Medicine, Rio Grande do Sul, Brazil.
- Department of Pathology, School of Medicine and Postgraduate Program in Dentistry, University of Passo Fundo, Passo Fundo, Rio Grande do Sul, Brazil.
- Department of Radiology, Affiliated Hospital of Atitus Education School of Medicine, Passo Fundo, Rio Grande do Sul, Brazil.
- Department of Neurology, Affiliated Hospital of Atitus Education School of Medicine, Passo Fundo, Rio Grande do Sul, Brazil.
Correspondence Address:
Augusto Müller Fiedler, Department of Neurological Surgery, University of Miami Hospital, Miami, Florida, United States.
DOI:10.25259/SNI_431_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Augusto Müller Fiedler1, Paulo Moacir Mesquita Filho2, Alessandra Loureiro Morassutti3, Robson Rottenfusser4, Daniel Lima Varela5. Primary central nervous system lymphoma in elderly: An illustrative case of the new role of surgery and integrative medical management. 01-Sep-2023;14:310
How to cite this URL: Augusto Müller Fiedler1, Paulo Moacir Mesquita Filho2, Alessandra Loureiro Morassutti3, Robson Rottenfusser4, Daniel Lima Varela5. Primary central nervous system lymphoma in elderly: An illustrative case of the new role of surgery and integrative medical management. 01-Sep-2023;14:310. Available from: https://surgicalneurologyint.com/surgicalint-articles/12521/
Abstract
Background: Primary central nervous system lymphoma (PCNSL) is a rare, aggressive non-Hodgkin lymphoproliferative neoplasm. Surgery is traditionally limited to biopsy due to past studies, but recent strong evidence continues to challenge this status quo in selected patients. Here, the authors characterize a case to illustrate the potential role of surgery and foster research on integrative medical management approaches for this disease.
Case Description: A 73-year-old woman was admitted to the hospital with aphasia and confusion. Neuroimaging suggested a lymphoproliferative process. The patient underwent cytoreductive surgery to resect the lesion. Microscopically, large infiltrating lymphoid cells that induced brain tissue damage were observed, and a diagnosis of diffuse large B-cell lymphoma was made based on immunohistochemistry. The patient evolved clinically post surgery. A complete response to further chemotherapy maintained the patient’s clinical recovery.
Conclusion: This rare case highlights the potential of surgical intervention in the management of selected patients with PCNSL. The authors also underscore the recent, meta-analytic evidence on surgery followed by combined chemotherapy for the management of specific cases. The reported recovery in an elderly patient is noteworthy and adds to the literature on this rare subtype of brain tumors. Future research should consider investigating a potential profile of candidates for resection and combined chemotherapy in PCNSL.
Keywords: Brain tumor, Diffuse large B-cell lymphoma (DLBCL), Neuro-oncology, Non-Hodgkin lymphoma (NHL), Primary central nervous system lymphoma (PCNSL)
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive non-Hodgkin lymphoproliferative neoplasm that swiftly infiltrates the brain, with a poor prognosis and limited treatment options. Over the years, the role of surgery in PCNSL has been a subject of debate. Historically, cytoreductive surgery was not a first-line approach due to concerns about the tumor’s diffuse nature, potential dissemination, and the belief that systemic chemotherapy alone would suffice. Resection has been typically discouraged in PCNSLs since 20th-century studies fostered the notion that the inherent risks outweigh the potential benefits. The tumor’s deep location or proximity to critical brain structures often made resection challenging without compromising neurological function. However, while chemotherapy has contributed to significant improvements in the management of PCNSL, these patients’ median overall survival (OS) rate is still only marginally better than that of glioblastomas (GBM).
Recent evidence, illustrated by the case we discuss herein, has begun to challenge the traditional stance, suggesting a potential role for surgical intervention – particularly if the tumor is exerting significant mass effect or is accessible – followed by combined chemotherapy with better outcomes in select cases. This report presents a 73-year-old woman with PCNSL who underwent symptomatic and diagnostic tumor resection, leading to the diagnosis of diffuse large B-cell lymphoma (DLBCL) based on immunohistochemistry. The patient recovered clinically post surgery and achieved a complete response with combined chemotherapy. Our report highlights the potential of an integrative medical management approach and underscores the urgency for further research on multimodal treatment options to determine a possible profile for such PCNSL patients.
CASE PRESENTATION
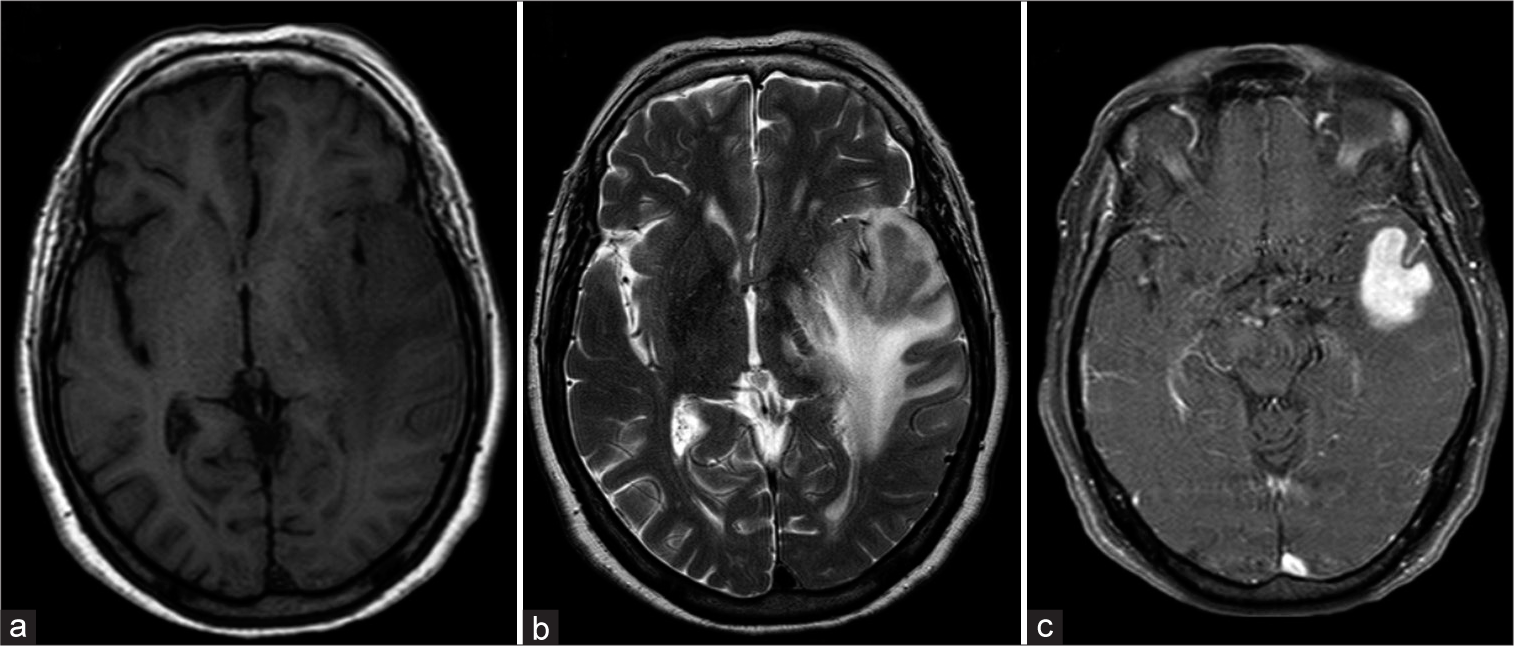
A 73-year-old, right-handed Caucasian woman, presented to the emergency department with a 1-week history of confusion, aphasia, and fluctuating consciousness. She experienced a decline in mental status and headaches the day before. Neurological examination showed a disoriented, cooperative female with severe mixed expression aphasia and a left extensor plantar cutaneous reflex. Past medical history was positive for hypertension, type 2 diabetes mellitus, and dyslipidemia, and the patient had no previous cardiovascular events. Non-contrast head CT showed a median shift to the right and suggestive findings of brain edema. Magnetic resonance imaging (MRI) revealed a 3.3 × 2.4 cm lesion in the left temporal lobe with hyposignal on T2, an intermediate signal on T1, diffusion restriction, and intense homogeneous contrast enhancement [
Figure 3:
(a-c) Control magnetic resonance imaging 2-month post-surgery: left pterional craniotomy with extra-axial residual blood collection. Partially resected infiltrative left temporal lesion with extension to the frontal and parietal lobes. Persistent contrast-enhancing lesion with mass effect on the left lateral ventricle.
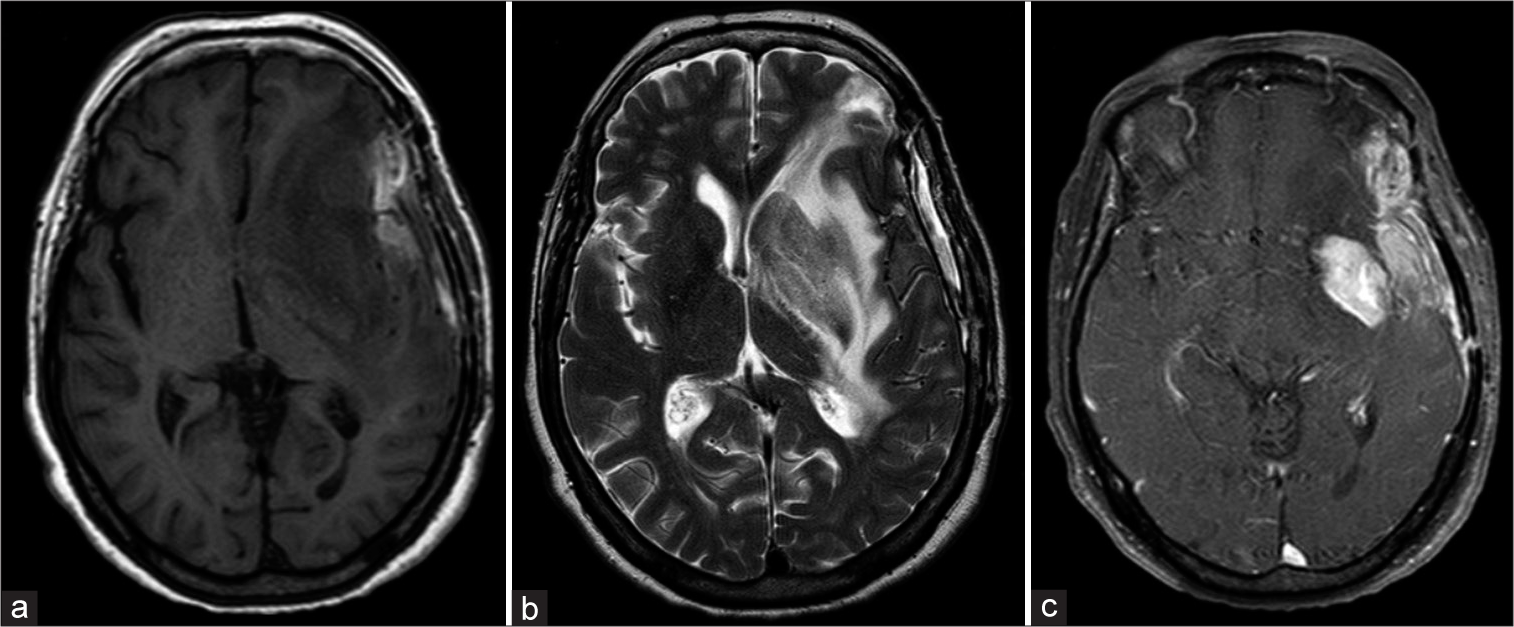
Figure 4:
(a-c) Control magnetic resonance imaging after two cycles of combined chemotherapy: left pterional craniotomy with extra-axial residual surgical hemostatic material (red arrows). Extensive parenchymal left temporal resection with extension into the frontal and parietal lobes. No contrast-enhancing lesion or midline shift.
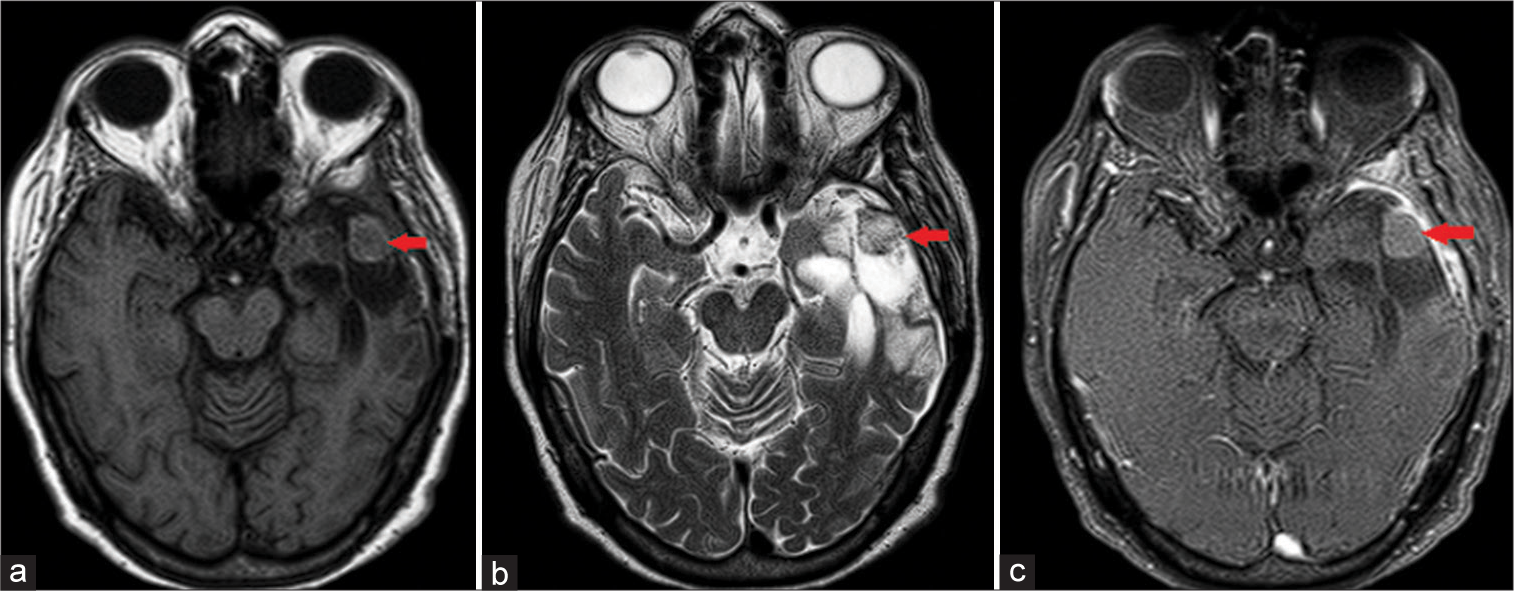
Figure 5:
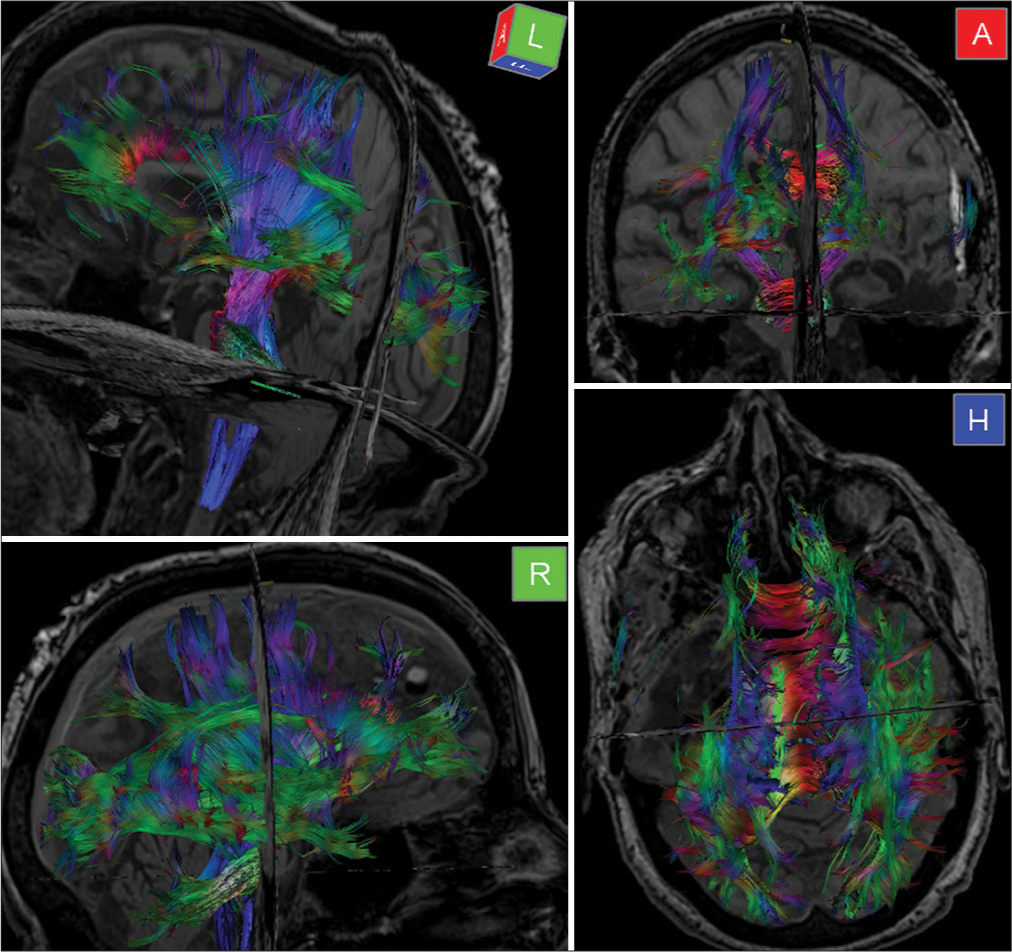
Diffusion tensor imaging and fiber tractography: marked reduction of fractional anisotropy (FA) in the left inferior longitudinal fasciculus and anterior corona radiata, suggesting fiber injury. Slight reduction of FA in the corpus callosum. No evidence of intracranial expansile process, hemorrhage, extra-axial collections, or midline shift.
DISCUSSION
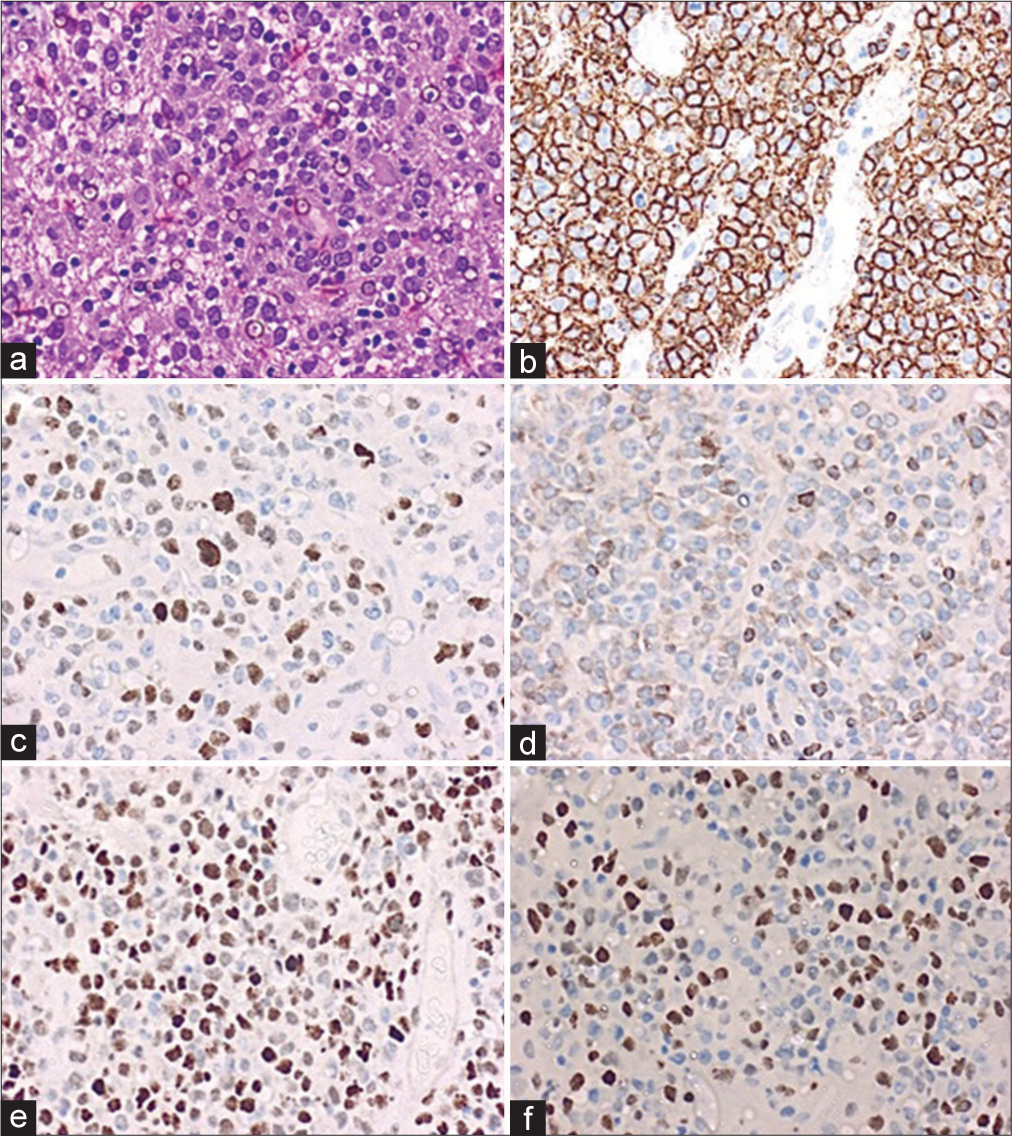
PCNSLs represent an aggressive subtype of non-Hodgkin lymphoma (NHL), originating within the brain, spinal cord, and eyes. They are predominantly characterized by their histological features, with DLBCL being the most common type. This central nervous system (CNS)-DLBCL subtype is composed of large, abnormal lymphocytes that rapidly proliferate and infiltrate the brain, often detected at high-grade stages.[
The histological profile is pivotal in guiding the treatment approach and determining the patient’s prognosis. Recognized by the 2021 World Health Organization classification of hematopoietic and lymphoid tissues, as well as by that of primary brain neoplasms, these lymphomas are nearly always (~95%) classified as CD20-positive DLBCL.[
PCNSLs are most common in immunocompetent elderly individuals, with incidence increasing with age. They constitute 2% of all brain tumors and 4–6% of NHLs. In the general population, the incidence is 0.5–1/100,000 individuals.[
The clinical presentation can vary depending on their location. PCNSLs typically exhibit a diffuse distribution, with the most common sites being the hemispheres (38%), that is, the frontoparietal, followed by the temporal lobe, basal ganglia (16%), corpus callosum (14%), periventricular regions (12%), and rarely the cerebellum (9%).[
The diagnosis of PCNSLs requires a brain MRI and biopsy. Radiological findings often show well-defined mass lesions that are hypointense on T1- and hyperintense on T2-weighted images, enhanced after contrast administration, with abundant edema.[
Moreover, a CSF analysis was precluded due to the imminent risk of brain herniation, as indicated by the significant midline shift and extensive brain edema seen in the patient’s MRI.[
Immunohistopathology of PCNSLs can indicate aggressiveness and invasiveness. DLBCL is the most common subtype with the least favorable prognosis.[
Surgery is essential for diagnosing PCNSL, and while the standard treatment is chemotherapy,[
The optimal management of PCNSL is still under debate but typically involves MTX-based chemotherapy.[
In the intricate landscape of PCNSL management, our case illustrates the efficacy of a comprehensive medical approach. The choice between biopsy and resection is pivotal.[
Elderly patients with PCNSL have a poor survival rate, with a median OS of 6–10 months and complete response rates at approximately 50%.[
This report highlights the benefits of resection in selected cases. Ten months after the hospital admission, surgery could provide symptomatic relief and preserved function in the severe, acute setting of an extensive lesion in noble brain areas. Combined with chemotherapy, this integrative approach led to a complete radiological and sustained clinical response. Future clinical trials should aim to investigate and determine the best-combined treatment modalities for this rare disease and potential eligibility criteria.[
CONCLUSION
The article describes a 73-year-old woman with PCNSL presenting with acute mixed expression aphasia and confusion. The patient underwent resection followed by chemotherapy, which led to a rapid and sustained recovery. The authors emphasize the unexplored potential of surgical resection to improve outcomes in selected PCNSL patients, which follows recent meta-analytic evidence. The findings suggest that the combined treatment of surgery and multiagent chemotherapy could successfully treat PCNSL while promoting preserved function and prolonged survival of certain patients. Overall, this report adds to the growing literature on the management of PCNSL in the elderly and fosters future research into multimodal therapies for this aggressive neoplasm.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review.
References
1. Bravetti C, Degaud M, Armand M, Sourdeau E, Mokhtari K, Maloum K. Combining MYD88 L265P mutation detection and clonality determination on CSF cellular and cell-free DNA improves diagnosis of primary CNS lymphoma. Br J Haematol. 2023. 201: 1088-96
2. Chojak R, Koźba-Gosztyła M, Polańska K, Rojek M, Chojko A, Bogacz R. Surgical resection versus biopsy in the treatment of primary central nervous system lymphoma: A systematic review and meta-analysis. J Neurooncol. 2022. 160: 753-61
3. Franca RA, Travaglino A, Varricchio S, Russo D, Picardi M, Pane F. HIV prevalence in primary central nervous system lymphoma: A systematic review and meta-analysis. Pathol Res Pract. 2020. 216: 153192
4. Guha A, Goda JS, Dasgupta A, Mahajan A, Halder S, Gawde J. Classifying primary central nervous system lymphoma from glioblastoma using deep learning and radiomics based machine learning approach-a systematic review and meta-analysis. Front Oncol. 2022. 12: 884173
5. Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015. 16: e322-32
6. Hoang-Xuan K, Deckert M, Ferreri AJ, Furtner J, Perez-Larraya J, Henriksson R. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro Oncol. 2023. 25: 37-53
7. Horbinski C, Nabors LB, Portnow J, Baehring J, Bhatia A, Bloch O. NCCN guidelines® insights: Central nervous system cancers, version 2.2022. J Natl Compr Canc Netw. 2023. 21: 12-20
8. Houillier C, Soussain C, Ghesquières H, Soubeyran P, Chinot O, Taillandier L. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology. 2020. 94: e1027-39
9. Huntoon K, Makary MS, Shah VS, Aquino A, Pandya V, Giglio P. Pretreatment findings on magnetic resonance imaging in primary central nervous system lymphoma may predict overall survival duration. Neuroradiol J. 2023. 36: 479-85
10. Kerbauy MN, Moraes FY, Lok BH, Ma J, Kerbauy LN, Spratt DE. Challenges and opportunities in primary CNS lymphoma: A systematic review. Radiother Oncol. 2017. 122: 352-61
11. Krishna S, Kakaizada S, Almeida N, Brang D, Hervey-Jumper S. Central nervous system plasticity influences language and cognitive recovery in adult glioma. Neurosurgery. 2021. 89: 539-48
12. Kurz KS, Ott G. The 5th edition of the WHO classification of lymphoid neoplasms-an overview. Pathologie (Heidelb). 2022. 43: 64-70
13. Li X, Huang Y, Bi C, Yuan J, He H, Zhang H. Primary central nervous system diffuse large B-cell lymphoma shows an activated B-cell-like phenotype with co-expression of C-MYC, BCL-2, and BCL-6. Pathol Res Pract. 2017. 213: 659-65
14. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, FigarellaBranger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
15. Morales H. Current and future challenges of functional MRI and diffusion tractography in the surgical setting: From eloquent brain mapping to neural plasticity. Semin Ultrasound CT MR. 2021. 42: 474-89
16. Morell AA, Shah AH, Cavallo C, Eichberg DG, Sarkiss CA, Benveniste R. Diagnosis of primary central nervous system lymphoma: A systematic review of the utility of CSF screening and the role of early brain biopsy. Neurooncol Pract. 2019. 6: 415-23
17. Mo SS, Cleveland J, Rubenstein JL. Primary CNS lymphoma: Update on molecular pathogenesis and therapy. Leuk Lymphoma. 2023. 64: 57-65
18. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022. 24: v1-95
19. Yamaguchi J, Ohka F, Kitano Y, Maeda S, Motomura K, Aoki K. Rapid detection of the MYD88 L265P mutation for preand intra-operative diagnosis of primary central nervous system lymphoma. Cancer Sci. 2023. 114: 2544-51
20. Yu J, Du H, Ye X, Zhang L, Xiao H. High-dose methotrexate-based regimens and post-remission consolidation for treatment of newly diagnosed primary CNS lymphoma: Meta-analysis of clinical trials. Sci Rep. 2021. 11: 2125