- Department of Neurosurgery, Hospital Prof Juan P. Garrahan, Buenos Aires, Argentina.
Correspondence Address:
Yamila Basilotta Marquez, Department of Neurosurgery, Hospital Prof Juan P. Garrahan, Buenos Aires, Argentina.
DOI:10.25259/SNI_641_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Marquez YB, Johnson AR, Argañaraz R, Mantese B. Primary diffuse leptomeningeal melanomatosis: A case report of an unusual presentation in a pediatric patient. Surg Neurol Int 12-Jan-2024;15:6
How to cite this URL: Marquez YB, Johnson AR, Argañaraz R, Mantese B. Primary diffuse leptomeningeal melanomatosis: A case report of an unusual presentation in a pediatric patient. Surg Neurol Int 12-Jan-2024;15:6. Available from: https://surgicalneurologyint.com/surgicalint-articles/12707/
Dear Editor,
Melanocytic tumors that involve the central nervous system may be primary or metastatic. Primary melanocytic tumors of the meninges are extremely rare.[
This type of neoplasm is rare in adults but even more so in children.[
CASE REPORT
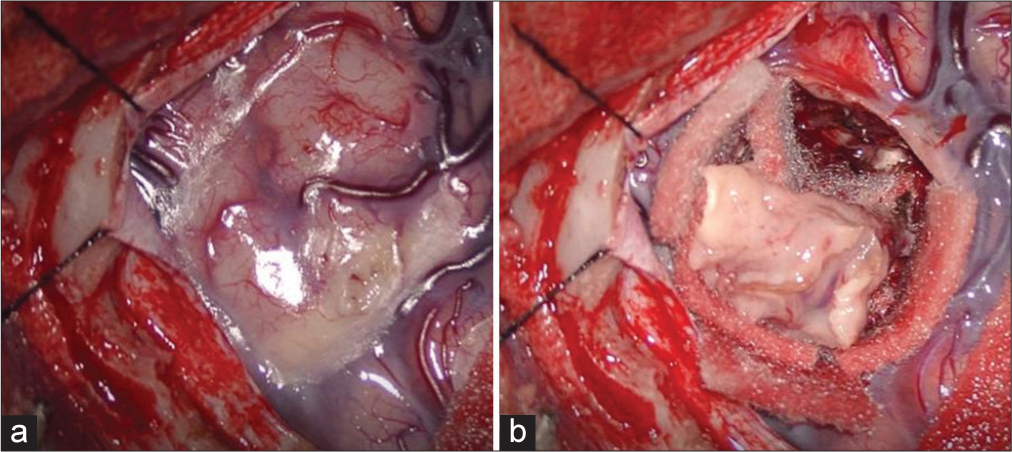
A 14-year-old female patient with no relevant history of disease presented to the hospital due to several days of headache associated with vomiting and photophobia. Brain computed tomography (CT) without contrast was performed, and no abnormalities were observed. The symptoms persisted, and the patient developed limb paresthesia. Fundoscopy showed bilateral papilledema. Magnetic resonance imaging (MRI) of the brain and spine showed interhemispheric leptomeningeal inflammation with intense contrast enhancement without ventricular enlargement. Lumbar puncture showed elevated cerebrospinal fluid (CSF) pressure. Tuberculous meningitis was suspected, and empirical antitubercular treatment was started. The tumor marker disialoganglioside GD2 was positive on CSF flow cytometry, and therefore, it was decided to perform a biopsy. For the biopsy, an interhemispheric approach with a left frontal craniotomy was used. A wedge specimen was taken from the leptomeninges and brain, and macroscopically, no pigmented area was observed [
Histopathological analysis showed neoplastic proliferation of cells with round nuclei and scant cytoplasm, with focal areas of brownish pigment. Immunohistochemistry was positive for HMB45 and variable for Ki67, which was higher in nests of intracortical tumor infiltration. PDLM was diagnosed.
The patient developed hydrocephalus, and a ventriculoperitoneal shunt was placed. Two shunt revisions were required due to obstruction of the proximal catheter. Temozolomide and dexamethasone were given. Subsequently, the patient started with seizures that were difficult to manage. The last brain CT scan revealed more lesions with marked perilesional edema. Due to the impossibility of curative treatment, palliative care was administered. She died four months after diagnosis.
DISCUSSION
The World Health Organization classifies primary leptomeningeal melanocytic neoplasms into circumscribed and diffuse lesions.[
Our patient presented with nonspecific symptoms, consistent with other reports in the literature. Imaging studies showed an interhemispheric pattern of contrast enhancement with little involvement of the basal cisterns. The initial presumptive diagnosis was tuberculous meningitis since this is an endemic disease in our environment. The presence of hyperintense signals in T1-weighted sequences due to the paramagnetic properties of melanin is helpful in the differential diagnosis.[
CSF samples were positive for disialoganglioside GD2, prompting biopsy. This type of sphingolipid is associated with tumor development and malignant phenotype.[
At the time of surgery, macroscopic examination did not reveal any pigmented areas. The patient also did not present skin lesions suggestive of a melanocytic cell-derived tumor. As these tumors originate from darkly pigmented cells (melanin), most of them are blackish in appearance. In our case, these features were not evident during surgery; therefore, we believe this may have been a non-pigmented variant.
Given that PDLM may mimic a wide variety of conditions, early diagnosis is important.[
Hydrocephalus generally occurs in the late stages of the disease.[
The behavior of these tumors is aggressive, and survival is <1 year after diagnosis. Treatments with different drugs and radiotherapy have been tried, but currently, there is no curative therapy.[
CONCLUSION
PDLM is a neoplastic disease with a poor prognosis for which no curative treatment has been found so far. Here, we report a pediatric patient with a macroscopically nonpigmented PDLM that presented a poor outcome in spite of medical treatment. We believe that CSF levels of tumor markers such as GD2 may aid in the diagnosis, excluding infections. Further study of this disease is important to improve therapeutic options and thereby prolong survival.
Ethical approval
Not applicable.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Angelino G, De Pasquale MD, De Sio L, Serra A, Massimi L, De Vito R. NRAS (Q61K) mutated primary leptomeningeal melanoma in a child: Case presentation and discussion on clinical and diagnostic implications. BMC Cancer. 2016. 16: 512
2. Aslan S, Gocmen R, Acar NP, Khasiyev F, Gumeler E, Soylemezoglu F. Two cases of primary leptomeningeal melanomatosis mimicking subacute meningitis. Neuroradiol J. 2018. 31: 42-6
3. Baumgartner A, Stepien N, Mayr L, Madlener S, Dorfer C, Schmook MT. Novel insights into diagnosis, biology and treatment of primary diffuse leptomeningeal melanomatosis. J Pers Med. 2021. 11: 292
4. Celli P, Acqui M, Trillò G, Ramundo EO, D’Andrea G, Roperto R. Primary leptomeningeal melanomatosis: Early leptomeningeal enhancement on MRI. J Neurosurg Sci. 2001. 45: 235-40
5. Kelsey PNRWA. Primary malignant melanoma of meninges_ atypical presentation of subacute meningitis. Pediatr Neurol. 1995. 12: 172-4
6. Küsters-Vandevelde HV, Küsters B, van Engen-van Grunsven AC, Groenen PJ, Wesseling P, Blokx WA. Primary melanocytic tumors of the central nervous system: A review with focus on molecular aspects. Brain Pathol. 2015. 25: 209-26
7. Lee HJ, Ahn BC, Hwang SW, Cho SK, Kim HW, Lee SW. F-18 fluorodeoxyglucose PET/CT and post hoc PET/MRI in a case of primary meningeal melanomatosis. Korean J Radiol. 2013. 14: 343-9
8. Misir Krpan A, Rakusic Z, Herceg D. Primary leptomeningeal melanomatosis successfully treated with PD-1 inhibitor pembrolizumab: A case report. Medicine (Baltimore). 2020. 99: e22928
9. Nazha B, Inal C, Owonikoko TK. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. 2020. 10: 1000
10. Nicolaides P, Newton RW, Kelsey A. Primary malignant melanoma of meninges: Atypical presentation of subacute meningitis. Pediatr Neurol. 1995. 12: 172-4
11. Padilla-Vázquez F, Escobar-de la Garma VH, AyalaArcipreste A, Mendizábal-Guerra R, Cuesta-Mejía T. Melanocitoma y melanomatosis meníngea, lesiones similares pero diferentes [Melanocytoma and meningeal melanocytosis, similar but different lesions]. Cir Cir. 2017. 85: 273-8
12. Palacka P, Slopovsky J, Makovnik M, Kajo K, Obertova J, Mego M. A case report of a patient with inoperable primary diffuse leptomeningeal melanomatosis treated with whole-brain radiotherapy and pembrolizumab. Medicine (Baltimore). 2022. 101: e28613
13. Szathmari A, Perbet R, Hermier M, Di Rocco F, Frappaz D, Mottolese C. Primary amelanotic leptomeningeal melanomatosis in a child: A rare but severe disease. World Neurosurg. 2016. 92: 581.e15-20
14. Tavana Rad S, Ashrafzadeh F, Golmakani H, Ganjeifar B. Nonsyndromic primary diffuse leptomeningeal melanomatosis in a child. Iran J Child Neurol. 2021. 15: 153-7
15. Xu X, Zheng Y, Li J, Wang F, Li F. Pediatric primary diffuse leptomeningeal melanomatosis: Case report and review of the literature. Medicine (Baltimore). 2020. 99: e19178