- Department of Neurosurgery, Sanford School of Medicine, Sioux Falls, South Dakota, United States
- Department of Pediatric Oncology, Sanford School of Medicine, Sioux Falls, South Dakota, United States.
Correspondence Address:
Dallas J. Soyland, Department of Neurosurgery, Sanford School of Medicine, Sioux Falls, South Dakota, United States.
DOI:10.25259/SNI_1172_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dallas J. Soyland1, Paul F. Thanel1, Meaghan E. Sievers1, Kayelyn Wagner2, Shawn M. Vuong1. Primary epidural sporadic Burkitt lymphoma in a 3-year-old: Case report and literature review. 25-Mar-2022;13:106
How to cite this URL: Dallas J. Soyland1, Paul F. Thanel1, Meaghan E. Sievers1, Kayelyn Wagner2, Shawn M. Vuong1. Primary epidural sporadic Burkitt lymphoma in a 3-year-old: Case report and literature review. 25-Mar-2022;13:106. Available from: https://surgicalneurologyint.com/surgicalint-articles/11482/
Abstract
Background: Burkitt lymphoma (BL) is a common tumor of childhood that usually arises in the abdomen or pelvis in its sporadic form. In a minority of cases, BL can present with CNS involvement, usually as a secondary site. Rarely, BL can arise primarily in the epidural space and present with back pain, or less commonly, acute myelopathy. This presentation is a surgical emergency and requires vigilant management.
Case Description: We describe a case of pediatric BL arising primarily within the epidural space and presenting with progressive difficulty walking in a 3-year-old boy. Progression to complete inability to walk, absent lower extremity deep tendon reflexes, and new urinary incontinence prompted MRI of the spine, which showed a lesion extending from T5 to T10 and wrapping around the anterior and posterior portions of the spine with evidence of spinal cord compression. The patient underwent decompressive laminectomies from T5 to T10 and partial debulking of the posterior portions of the tumor. Microscopic examination showed a prominent “starry sky” pattern with abundant mitotic figures. Immunohistochemistry confirmed the diagnosis of BL. The patient is 10 months post-op and continues to undergo chemotherapy with partial neurologic improvement. He was free of recurrence 10 months post-operative.
Conclusion: This appears to be the youngest described patient presenting with acute myelopathy in primary paraspinal BL. Management should include surgical decompression of the spinal cord followed by one of the various described chemotherapeutic regimens. Preoperative staging and neurologic function correlate with prognosis.
Keywords: Burkitt lymphoma, Pediatric lymphoma, Pediatric myelopathy, Pediatric paraspinal tumor, Spinal neoplasia
INTRODUCTION
Burkitt’s lymphoma (BL) is a common childhood tumor first described and eponymously named in 1958 by Denis Burkitt, after observing multiple children with jaw tumors in a Ugandan hospital.[
The three distinct subtypes of BL currently recognized include endemic, sporadic, and immunodeficiency-associated BL. Endemic BL is highly prevalent in malaria-endemic regions such as equatorial Africa, with ~3–6 cases per 100,000 children per year.[
BL occurs at a 10-fold lower incidence outside of malaria-endemic regions.[
Immunodeficiency-associated BL is prevalent among individuals with HIV as opposed to other causes of immunodeficiency.[
The presentation and primary tumor sites of BL vary between the demographic subtypes. Unlike the endemic form of the disease, which most commonly arises in the jaw, the sporadic form arises from the abdomen in 60–80% of cases.[
Pediatric epidural tumors causing spinal cord compression are rare and require emergent management. About 70% of childhood tumors causing spinal cord compression are extradural; lymphomas make up a minute percentage of the differential.[
CASE PRESENTATION
A 3-year-old Caucasian male presented to an outside hospital with 2 weeks of progressively worsening trouble walking. He was initially prescribed azithromycin and prednisone at an outside clinic for a presumed infectious etiology; however, his gait instability continued to worsen. Two weeks before presentation he had an unwitnessed fall down a flight of stairs without acute injury. Pelvic X-rays showed no irregularities and CBC and blood chemistry were normal. Progression to complete inability to walk as well as areflexia in the lower extremities and new-onset urinary incontinence prompted further workup, for which the patient was then transferred to University of South Dakota Sanford Sioux Falls Hospital.
On arrival, the patient was noted to have significant weakness in his lower extremities bilaterally and was uncooperative with physical examination. Strength was 0 out of 5 in the left and 1 out of 5 in the right lower extremity. Deep tendon reflexes and sensation to light touch were absent bilaterally. The patient had no pertinent medical, family, or social history. He did not have any history suggestive of immunocompromised. Further workup included a normal CBC and CRP. An MRI of the brain, as well as the cervical, thoracic, and lumbar spine were completed under anesthesia and revealed a large soft tissue mass extending in the epidural space from T5 to T10 [
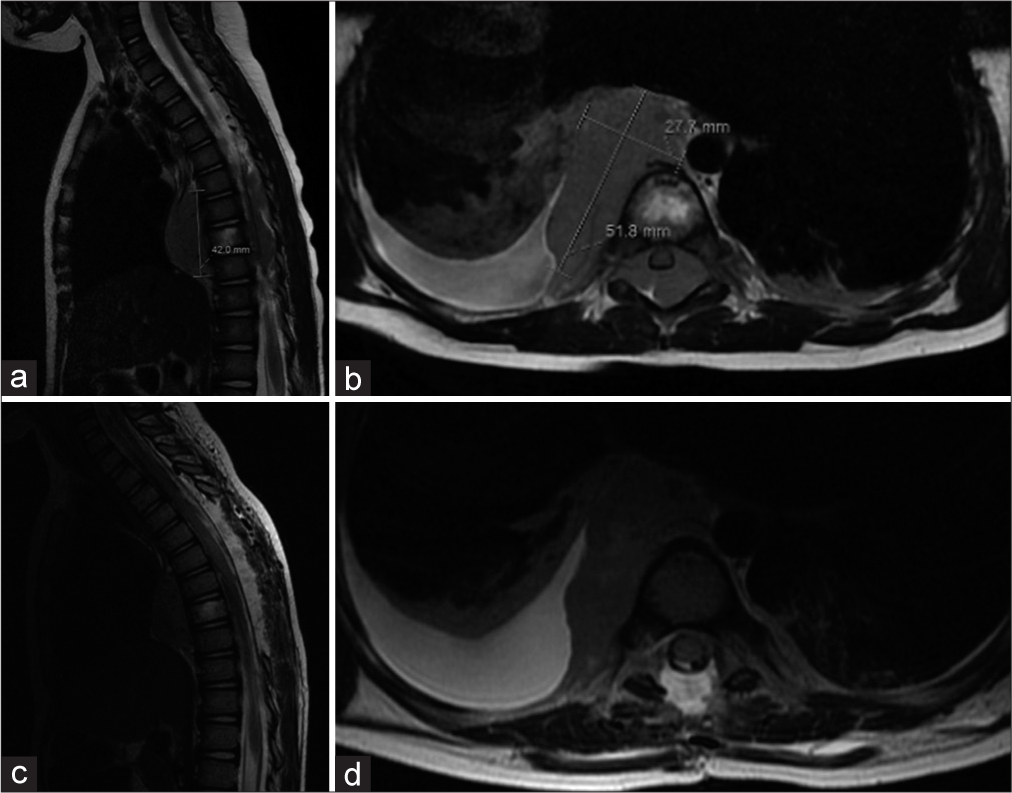
Figure 1:
Sagittal (a) and axial (b) T2-weighted MRI preoperative images show an extensive soft-tissue mass in the epidural space from T5 to T10 with involvement of the T8 vertebral body with extension anteriorly into the posterior mediastinum. Spinal cord compression can be seen in the axial view. Postoperative T2-weighted MRI (c and d) shows removal of laminae, debulking of the dorsal portion of the tumor, as well as resolution of the spinal cord compression.
Due to the rapidly progressive myelopathic course, the decision was made to perform an emergent decompression. The extension of the tumor to the anterior surface of the vertebral bodies made complete resection improbable, so we elected to remove only the posterior portions of the tumor to achieve acute symptomatic relief and obtain a histologic diagnosis, with a plan to address the remaining tumor after diagnosis was obtained. Complete T5-T9 laminectomies and a partial T10 laminectomy were carried out and the mass visualized as fatty-appearing homogenous tissue. The mass was also noted to protrude dorsally after removal of the bone tissue, suggesting compression before the procedure. Using microscopy, the lesion was subsequently dissected and removed from the dorsal aspect of the spinal cord dura and sent to pathology. Decompression of the cord was achieved. As planned, the lesion was not completely removed, as it occupied the anterior aspect of the spine as well as parts of the lateral canal. The procedure was well tolerated and without complication.
Histopathology revealed a prominent “starry sky” pattern, the classic description for marked apoptosis with large amounts of tingible body macrophages. High mitotic activity and foci of necrosis were present. Flow cytometry revealed the cells to express CD45, CD20, CD10, CD19, and CD38. CD34 and TdT were not expressed. The cells expressed high levels of c-MYC (80–90%). These findings indicated a high-grade B-cell lymphoma, strongly suggestive of Burkitt lymphoma (BL). Paraffin block confirmed c-MYC/IGH fusion through fluorescence in situ hybridization panel, further confirming the diagnosis.
Initial chemotherapy included intrathecal methotrexate and hydrocortisone. He is currently undergoing a regimen according to ANHL1131 with COP-R reduction phase including cyclophosphamide, vincristine, prednisolone, and rituximab. His course has been complicated by bacteremia and mucositis with perirectal ulceration, which delayed his chemotherapeutic schedule temporarily and required diverting colostomy; however, his clinical picture is overall improving. He has regained walking ability, lower extremity deep tendon reflexes, and sensation to light touch. He has continued to have some postoperative neuropathy of the lower extremities that is under control with gabapentin. Positron emission tomography 3 months post-op showed regression of the tumor compatible with response to chemotherapy. Repeat imaging 9 months post-op showed no evidence of residual tumor or metastasis. The patient is now 10 months post-op and has returned home after intensive inpatient physical therapy and social interventions.
DISCUSSION
Although CNS involvement is relatively common in BL at the time of diagnosis, compressive myelopathy as the presenting symptom is rare observed in less than 3% of patients with NHL.[
BL is generally divided into three groups based on patient demographics: endemic (African), immunodeficiency-associated, and sporadic. One retrospective study found that BL accounted for 30% of pediatric lymphomas and 40% of NHL in the United States with an incidence of three cases per million persons per year.[
The presentation and symptomatology of BL are highly dependent on the epidemiologic subtype. Pediatric sporadic BL most commonly presents in the abdomen (60–80%), followed by the head and neck.[
The pathogenesis of BL classically involves the translocation of the c-myc gene on chromosome 8 to another locus that undergoes prolific transcription in mature B cells, most commonly nearby the IgH enhancer on chromosome 14.[
Paravertebral tumors constitute just 4.8% of pediatric cancers.[
The classical morphology of BL is described as a “starry sky” appearance, composed of atypical lymphoid cells interspersed with tingible body macrophages ingesting the remnants of apoptotic cells due to the rapid growth of the tumor exceeding vasculogenesis.[
Treatment for epidural BL differs from typical recommendations due to the emergent need to decompress the spinal cord. In a patient who presents with compressive myelopathy, emergent multilevel decompressive laminotomy/laminectomy should be considered. Often, BL that either presents primarily in or metastasized to the epidural space involves the anterior portion of the spinal canal; in these cases, the goal of surgery must be to decompress the neuroaxis, with understanding that the remaining lymphoma is often sensitive to immunochemotherapy. Extraction of the posterior portions of the tumor also allows for pathologic diagnosis, which further guides treatment. Although some authors have suggested that laminotomy or laminoplasty may be preferred to laminectomy to avoid subsequent spinal instability, one review showed no difference in long-term function or deformity between epidural BL patients treated with laminectomy and those treated with laminotomy.[
Immunochemotherapeutic regimens for BL often vary between institutions, but most commonly include rituximab, a monoclonal antibody directed against CD-20, to target B-cells. Historically, the BL regimen followed that of acute lymphoblastic leukemia, meaning long, and intense cycles. This was not found to be effective in BL and does not target the unique behavior of the tumor. Because BL exhibits such rapid growth, prolonged courses of chemotherapy are likely to promote re-entrance of malignant cells into the cell cycle in between regimen cycles, causing drug resistance. For this reason, regimens were developed specifically for BL that had shortened durations of about 48–72 h; these were found to be more efficacious.[
CONCLUSION
BL is a common childhood malignancy. Sporadic BL occurs in immunocompetent hosts from non-endemic areas, and most commonly arises within the abdomen or pelvis. BL arising primarily in the epidural space and presenting as acute compressive myelopathy in a pediatric patient is exceedingly rare. To the best of our knowledge, this is the youngest described patient with sporadic primary paraspinal BL. A paraspinal mass with epidural extension causing myelopathy is a surgical emergency. Chemotherapeutic regimens vary between institutions but should include neurotropic agents. Initial grade and neurologic status are important prognostic indicators.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: The next step. J Clin Oncol. 2000. 18: 3144-50
2. Atallah-Yunes SA, Murphy DJ, Noy A. HIV-associated Burkitt lymphoma. Lancet Haematol. 2020. 7: e594-600
3. Biko DM, Anupindi SA, Hernandez A, Kersun L, Bellah R. Childhood Burkitt lymphoma: Abdominal and pelvic imaging findings. AJR Am J Roentgenol. 2009. 192: 1304-15
4. Bishop PC, Rao VK, Wilson WH. Burkitt’s lymphoma: Molecular pathogenesis and treatment. Cancer Invest. 2000. 18: 574-83
5. Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004. 104: 3009-20
6. Boxer LM, Dang CV. Translocations involving c-myc and c-myc function. Oncogene. 2001. 20: 5595-610
7. Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958. 46: 218-23
8. Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007. 109: 2736-43
9. Cairo MS, Sposto R, Perkins SL, Meadows AT, Hoover-Regan ML, Anderson JR. Burkitt’s and Burkitt-like lymphoma in children and adolescents: A review of the Children’s Cancer Group experience. Br J Haematol. 2003. 120: 660-70
10. Casulo C, Friedberg JW. Burkitt lymphoma-a rare but challenging lymphoma. Best Pract Res Clin Haematol. 2018. 31: 279-84
11. Chapman CJ, Wright D, Stevenson FK. Insight into Burkitt’s lymphoma from immunoglobulin variable region gene analysis. Leuk Lymphoma. 1998. 30: 257-67
12. Chapman CJ, Zhou JX, Gregory C, Rickinson AB, Stevenson FK. VH and VL gene analysis in sporadic Burkitt’s lymphoma shows somatic hypermutation, intraclonal heterogeneity, and a role for antigen selection. Blood. 1996. 88: 3562-8
13. Daley MF, Partington MD, Kadan-Lottick N, Odom LF. Primary epidural burkitt lymphoma in a child: Case presentation and literature review. Pediatr Hematol Oncol. 2003. 20: 333-8
14. Davi F, Delecluse HJ, Guiet P, Gabarre J, Fayon A, Gentilhomme O. Burkitt-like lymphomas in AIDS patients: Characterization within a series of 103 human immunodeficiency virus-associated non-Hodgkin’s lymphomas. Burkitt’s Lymphoma Study Group. J Clin Oncol. 1998. 16: 3788-95
15. Derinkuyu BE, Boyunaga O, Oztunali C, Tekkesin F, Damar C, Alimli AG. Imaging features of Burkitt lymphoma in pediatric patients. Diagn Interv Radiol. 2016. 22: 95-100
16. Dho YS, Kim H, Wang KC, Kim SK, Lee JY, Shin HY. Pediatric spinal epidural lymphoma presenting with compressive myelopathy: A distinct pattern of disease presentation. World Neurosurg. 2018. 114: e689-97
17. Fassett DR, Clark R, Brockmeyer DL, Schmidt MH. Cervical spine deformity associated with resection of spinal cord tumors. Neurosurg Focus. 2006. 20: E2
18. Gerrard M, Cairo MS, Weston C, Auperin A, Pinkerton R, Lambilliote A. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: Results of the FAB/LMB 96 international study. Br J Haematol. 2008. 141: 840-7
19. Grande BM, Gerhard DS, Jiang A, Griner NB, Abramson JS, Alexander TB. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood. 2019. 133: 1313-24
20. Gregory MA, Hann SR. c-Myc proteolysis by the ubiquitinproteasome pathway: stabilization of c-Myc in Burkitt’s lymphoma cells. Mol Cell Biol. 2000. 20: 2423-35
21. Hecht JL, Aster JC. Molecular biology of Burkitt’s lymphoma. J Clin Oncol. 2000. 18: 3707-21
22. Hoyoux C, Forget P, Piette C, Dresse MF, Florkin B, Rausin L. Paravertebral Burkitt’s lymphoma in a child: An unusual presentation. Case Rep Med. 2012. 2012: 891714
23. Jacobson C, LaCasce A. How I treat Burkitt lymphoma in adults. Blood. 2014. 124: 2913-20
24. Jaffe ES. The 2008 WHO classification of lymphomas: Implications for clinical practice and translational research. Hematol Am Soc Hematol Educ Program. 2009. 1: 523-31
25. Kim YS, Lee JK, Choi KY, Jang JW. Spinal Burkitt’s lymphoma mimicking dumbbell shape neurogenic tumor: A case report and review of the literature. Korean J Spine. 2015. 12: 221-4
26. Koeller KK, Rosenblum RS, Morrison AL. Neoplasms of the spinal cord and filum terminale: Radiologic-pathologic correlation. Radiographics. 2000. 20: 1721-49
27. Kurucu N, Akyuz C, Varan A, Akcoren Z, Aydin B, Yalcin B. Primary paraspinal and spinal epidural non-hodgkin lymphoma in childhood. J Pediatr Hematol Oncol. 2021. 43: e395-400
28. Lukes RJ, Collins RD. New approaches to the classification of the lymphomata. Br J Cancer Suppl. 1975. 2: 1-28
29. Magrath IT. African Burkitt’s lymphoma. History, biology, clinical features, and treatment. Am J Pediatr Hematol Oncol. 1991. 13: 222-46
30. Matsubara H, Watanabe KI, Sakai H, Chang H, Fujino H, Higashi Y. Rapid improvement of paraplegia caused by epidural involvements of Burkitt’s lymphoma with chemotherapy. Spine (Phila Pa 1976). 2003. 29: 4-6
31. Mbulaiteye SM, Biggar RJ, Bhatia K, Linet MS, Devesa SS. Sporadic childhood Burkitt lymphoma incidence in the United States during 1992-2005. Pediatr Blood Cancer. 2009. 53: 366-70
32. Minard-Colin V, Auperin A, Pillon M, Burke GA, Barkauskas DA, Wheatley K. Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med. 2020. 382: 2207-19
33. Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G. Burkitt’s lymphoma. Lancet. 2012. 379: 1234-44
34. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006. 107: 265-76
35. Murphy SB, Magrath IT. Workshop on pediatric lymphomas: Current results and prospects. Ann Oncol. 1991. 2: 219-23
36. Pollono D, Tomarchia S, Drut R, Ibanez O, Ferreyra M, Cedola J. Spinal cord compression: A review of 70 pediatric patients. Pediatr Hematol Oncol. 2003. 20: 457-66
37. Preudhomme C, Dervite I, Wattel E, Vanrumbeke M, Flactif M, Lai JL. Clinical significance of p53 mutations in newly diagnosed Burkitt’s lymphoma and acute lymphoblastic leukemia: A report of 48 cases. J Clin Oncol. 1995. 13: 812-20
38. Pui CH, Dahl GV, Hustu HO, Murphy SB. Epidural spinal cord compression as the initial finding in childhood acute leukemia and non-Hodgkin lymphoma. J Pediatr. 1985. 106: 788-92
39. Rajz G, Cohen JE, Harnof S, Knoller N, Goren O, Shoshan Y. Spontaneous spinal epidural hematoma: The importance of preoperative neurological status and rapid intervention. J Clin Neurosci. 2015. 22: 123-8
40. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica. 2010. 102: 83-7
41. Saleh K, Michot JM, Camara-Clayette V, Vassetsky Y, Ribrag V. Burkitt and Burkitt-like lymphomas: A systematic review. Curr Oncol Rep. 2020. 22: 33
42. Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O. Incidence of hematologic malignancies in Europe by morphologic subtype: Results of the HAEMACARE project. Blood. 2010. 116: 3724-34
43. Sereke SG, Bongomin F, Muyinda Z. Primary intradural extramedullary spinal Burkitt’s lymphoma: A case report. Int Med Case Rep J. 2020. 13: 701-5
44. Tepper CG, Seldin MF. Modulation of caspase-8 and FLICE-inhibitory protein expression as a potential mechanism of Epstein-Barr virus tumorigenesis in Burkitt’s lymphoma. Blood. 1999. 94: 1727-37
45. Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004. 350: 1328-37
46. Ziegler JL. Burkitt’s lymphoma. N Engl J Med. 1981. 305: 735-45