- Department of Spine, MIOT Hospital, Chennai, Tamil Nadu, India.
DOI:10.25259/SNI_497_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anandkumar Khatavi, Charanjit Singh Dhillon, Nilay Chhasatia, Chetan Pophale, Shafeek Nanakkal, Amit Varshney. Primary Ewing’s sarcoma of the C2 vertebra with progressive quadriparesis: Report of a rare case and review of the literature. 15-Oct-2020;11:340
How to cite this URL: Anandkumar Khatavi, Charanjit Singh Dhillon, Nilay Chhasatia, Chetan Pophale, Shafeek Nanakkal, Amit Varshney. Primary Ewing’s sarcoma of the C2 vertebra with progressive quadriparesis: Report of a rare case and review of the literature. 15-Oct-2020;11:340. Available from: https://surgicalneurologyint.com/surgicalint-articles/10327/
Abstract
Background: Ewing’s sarcoma is a malignant primitive neuroectodermal tumor (PNET) of childhood and adolescence. Primary Ewing’s sarcoma of the spine is uncommon, and even more rarely involves the C2 vertebra.
Case Description: A 14-year-old patient was admitted with a history of chronic neck pain, which exacerbated after playing contact sports 3 weeks before presentation. On initial examination, he had pain radiating into the left upper extremity plus spasticity in all the four limbs. The cervical X-rays revealed a mixed sclerotic-lytic lesion involving the C2 vertebral body. The CT bony and soft-tissue windows documented predominant left-sided tumor invasion of the posterior elements, pedicles, and body of C2 along with extension into the spinal canal resulting in severe cord compression with peritumoral soft-tissue edema. The angiogram revealed a patent left vertebral artery entirely surrounded/encased by tumor. The PET-CT scan demonstrated no other spinal or systemic lesions. Due to his rapid neurological deterioration, the patient underwent an emergent biopsy of the tumor with posterior decompression and occipitocervical stabilization. The biopsy demonstrated a PNET (e.g., positive CD 99 MIC2 marker for Ewing’s sarcoma). Following subsequent chemotherapy and radiation, the patient rapidly improved over a period of 3 months.
Conclusion: Primary Ewing’s sarcoma involving the C2 vertebra is exceedingly rare and warrants surgical decompression with pathological confirmation to provide additional multi-modal/multi-disciplinary adjunctive radiation/chemotherapy.
Keywords: C2 vertebra, Cervical spine, Ewing’s sarcoma, Primitive neuroectodermal tumor
INTRODUCTION
Ewing’s sarcoma is a malignant, primitive neuroectodermal tumor that occurs in the pediatric or young adult population. It involves more males than females, and has a peak age incidence of 15.[
CASE DESCRIPTION
A 14-year-old male, who sustained a sports injury 3 weeks ago, acutely presented with progressive neck pain and left upper limb radiculopathy and quadriparesis. On examination, he had tenderness over the upper cervical spine, and exhibited a Nurick Grade III myelopathy characterized by weakness (Medical Research Council Grade 4/5) in all four extremities with spasticity in all four extremities.
Radiographic studies
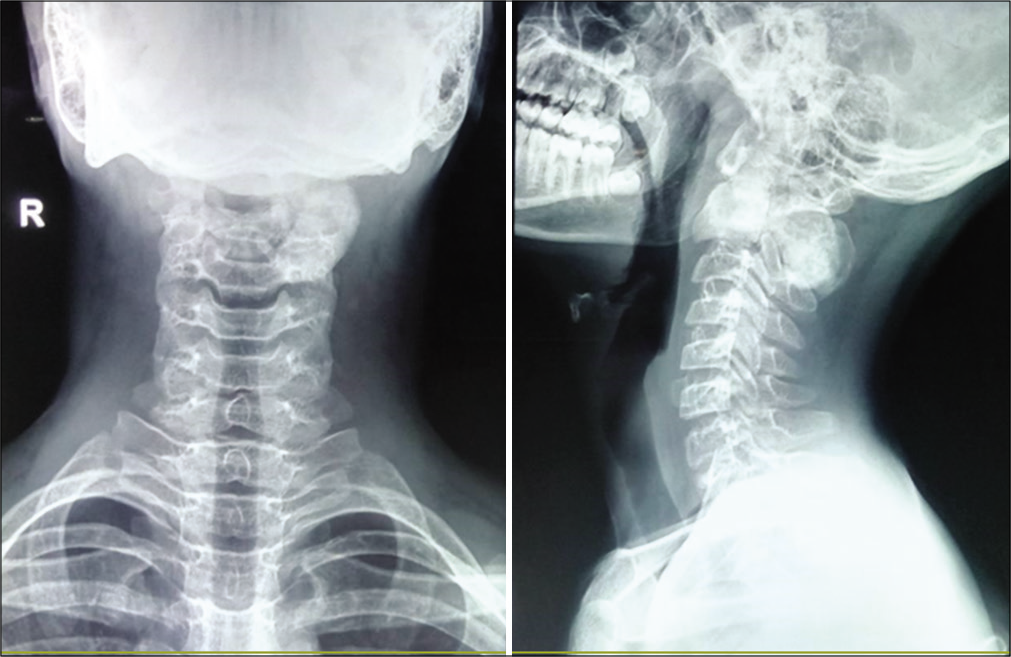
Radiographs of the cervical spine showed a predominantly sclerotic lesion with multiple lytic foci involving the C2 vertebra [
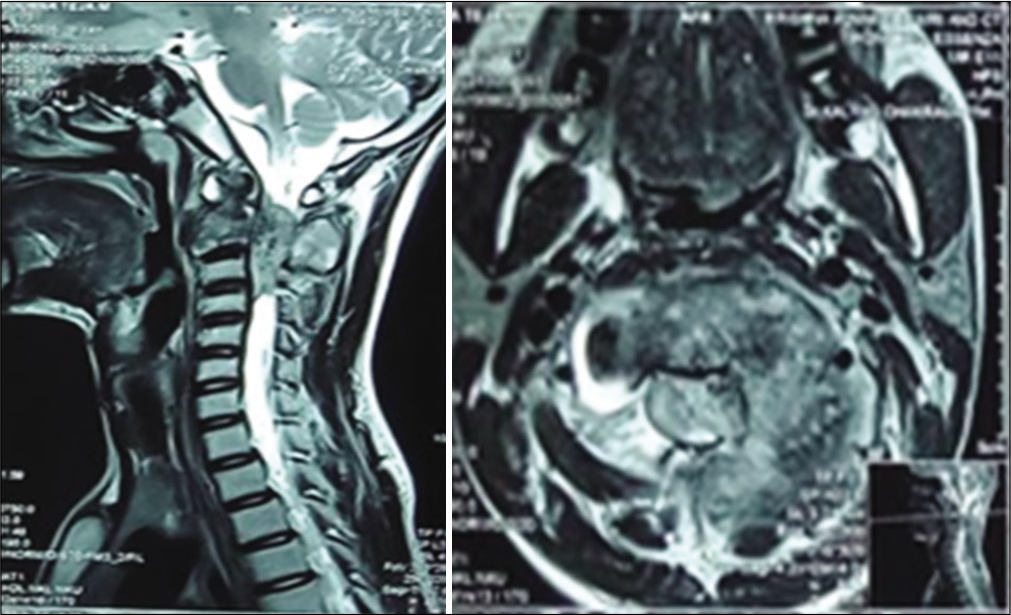
The MR revealed an expansile lesion involving the C2 vertebral body; the tumor infiltrated the laminae, pedicles, transverse processes, and spinous process [
Clinical and surgical course
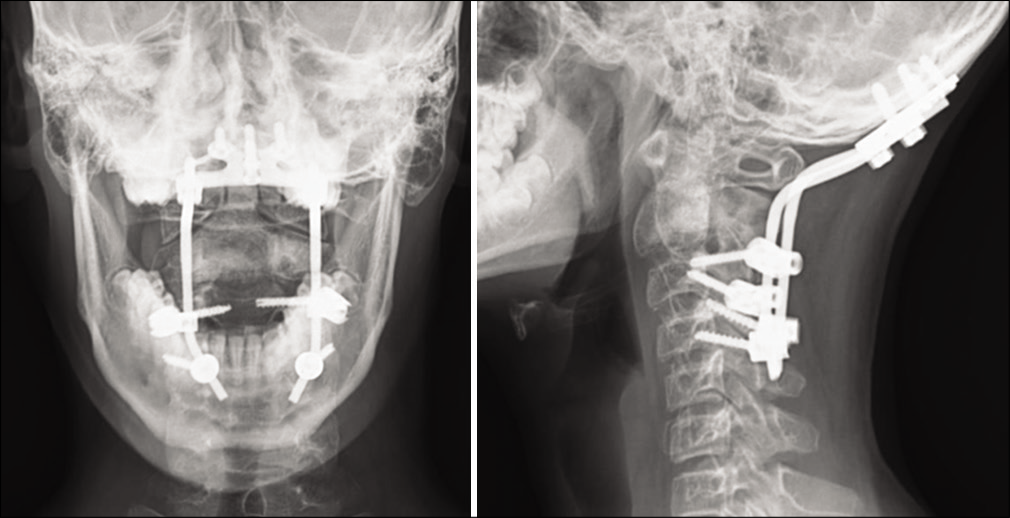
Over 2 days, the patient neurologically deteriorated, and lost sphincter functions. He, therefore, underwent a posterior spinal decompression (e.g., laminectomy C2/C3) with biopsy of the lesion and occipitocervical stabilization (C2-C4). Intraoperatively, the lesion appeared highly vascular with grayish white cheesy material destroying the neural arch, especially on the left side.
Surgical excision required; partial removal of the C2 vertebra (e.g., full resection was not deemed feasible because of encasement of the left vertebral artery), posterior excision of tumor involving the C2 lamina, and C3 superior dome laminectomy. Stabilization required placement of an occipital plate with pedicle screws placed at C3, and lateral mass screws at C4.
Pathology
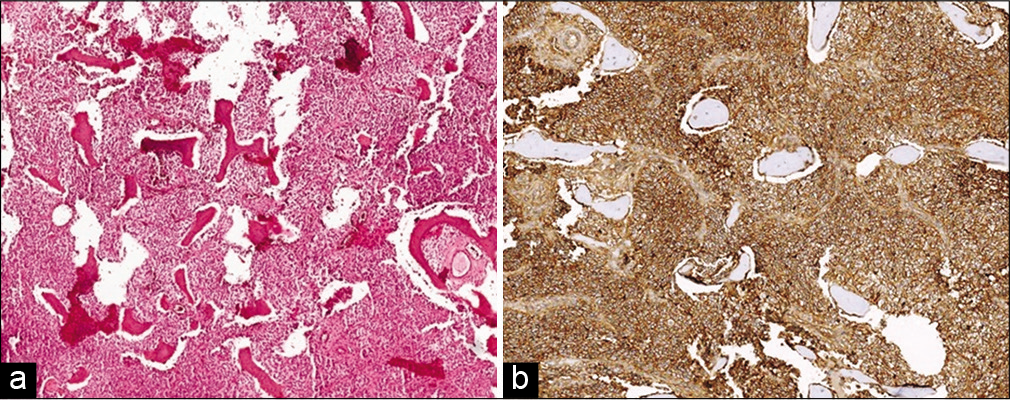
The frozen section diagnosis favored a round cell tumor. The final histopathology showed a densely cellular tumor consisting of small round cells with clear cytoplasm [
Postoperative course
Postoperatively, the patient underwent physiotherapy, rehabilitation, and chemotherapy (e.g., received vincristine, adriamycin, cyclophosphamide, ifosfamide, and etoposide according to National Copmrehensive Cancer Network guidelines).[
DISCUSSION
Frequency of spinal Ewing’s sarcomas
Ewing’s sarcoma involving the cervical spine, particularly involving the atlantoaxial junction, is rare, and constitutes approximately 3.2% of all spinal Ewing’s sarcomas.[
Differential diagnoses
The differential diagnoses of cervical Ewing’s sarcomas include giant cell tumor, aneurysmal bone cyst, Langerhans cell histiocytosis, vertebral hemangioma, chordoma, malignant lymphoma, metastatic embryonal rhabdomyosarcoma, neuroblastoma, and bacterial infection.[
Treatment options
The treatment of cervical Ewing’s sarcomas must be individualized based on the age, location, and size of the tumor, neurological deficits, reconstruction options, and experience of the surgeon. Postoperative chemotherapy is paramount in eliminating the residual tumor, and reducing the likelihood of metastasis. Giakoumettis et al.[
Surgical options
En-bloc excision at the occipitocervical junction presents a tremendous technical challenge. Liu et al.[
Although the axial location of a Ewing’s sarcoma is a poor prognostic factor,[
CONCLUSION
Primary Ewing’s sarcomas involving the C2 vertebrae are exceedingly rare. Optimal treatment typically includes adequate radiological evaluation, biopsy of the lesion to obtain immunohistopathological confirmation of the diagnosis, decompression where indicated, and postoperative multimodal adjunctive chemotherapy.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aggarwal AN, Chaturvedi S, Gulati D, Kumar S. Primary Ewing’s sarcoma of the second cervical vertebra: A rare entity. J Pediatr Orthop Part B. 2011. 20: 408-12
2. Ahmed R, Menezes AH. Primary atlantoaxial bone tumors in children: Management strategies and long-term follow-up: Clinical article. J Neurosurg Pediatr. 2014. 13: 260-72
3. Ahrens S, Dunst J, Exner GU, Kotz R, Paulussen M, Winkelmann W. Localized Ewing’s tumor of bone: Final results of the cooperative Ewing’s Sarcoma Study CESS 86. J Clin Oncol. 2001. 19: 1818-929
4. Burgers JM, Dunst J, Hawlicek R, Jurgens H, Michaelis J, Sauer R. Prognostic factors in the treatment of Ewing’s sarcoma. The Ewing’s sarcoma study group of the German society of paediatric oncology CESS 81. Radiother Oncol. 1987. 10: 101-10
5. El-Khoury GY, Haddad FS, Haddad SF, Hitchon PW, Sharafuddin MJ. Treatment options in primary Ewing's sarcoma of the spine. Neurosurgery. 1992. 30: 610-8
6. Giakoumettis D, Georgoulis G, Nikas I, Paraskeva K, Sfakianos G, Themistocleous MS. Primary Ewing sarcoma of the axis-C2: A case report and the review of the literature. Neurol Neurochir Pol. 2018. 52: 534-42
7. NCCN Clinical Practice Guidelines in Oncology. Available from: https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf [Last accessed on 2016 Aug 29].
8. Liu X, Wei F, Xu N, Jiang L, Cai H, Li Z. reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with ewing sarcoma. Spine (Phila Pa 1976). 2016. 41: E50-4
9. Stamenkovic I, Riggi N. The biology of Ewing sarcoma. Cancer Lett. 2007. 254: 1-10
10. Villas C, San Julian M. Ewing’s tumor of the spine: Report on seven cases including one with a 10-year follow-up. Eur Spine J. 1996. 5: 412-7