- Department of Neurosurgery Japanese Red Cross Otsu Hospital, Otsu, Japan
- Department of Hematology Japanese Red Cross Otsu Hospital, Otsu, Japan.
Correspondence Address:
Toshinari Kawasaki, Department of Neurosurgery, Japanese Red Cross Otsu Hospital, Otsu, Japan.
DOI:10.25259/SNI_792_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryo Hamamoto1, Toshinari Kawasaki1, Masashi Oda1, Sosuke Sumiyoshi1, Kosuke Hayashi1, Tamaki Kobayashi1, Yoshihiko Ioroi1, Tatsuki Uchiyama2, Motohiro Takayama1, Masaaki Saiki1. Primary extranodal marginal zone mucosa-associated lymphoid tissue-type B-cell lymphoma involving the dura: A case report. 29-Mar-2024;15:113
How to cite this URL: Ryo Hamamoto1, Toshinari Kawasaki1, Masashi Oda1, Sosuke Sumiyoshi1, Kosuke Hayashi1, Tamaki Kobayashi1, Yoshihiko Ioroi1, Tatsuki Uchiyama2, Motohiro Takayama1, Masaaki Saiki1. Primary extranodal marginal zone mucosa-associated lymphoid tissue-type B-cell lymphoma involving the dura: A case report. 29-Mar-2024;15:113. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12826
Abstract
Background: Primary extranodal marginal zone mucosa-associated lymphoid tissue-type B-cell lymphoma (EMZMBCL), which presents as a dural mass, is a rare intracranial tumor that mimics a subdural hematoma or meningioma.
Case Description: A 49-year-old woman presented to our hospital with transient right upper limb paresis, dysarthria for 10 min, and ongoing right upper-limb numbness. Computed tomography (CT) of the head revealed extra-axial lesions in the left frontal and parietal lobes. Based on the initial CT findings in the emergency room, an acute subdural hematoma was suspected. However, meningiomas and other intracranial tumors were also listed as differential diagnoses because there was no history of head trauma or coagulation abnormalities on blood examination, and further imaging studies were performed. Imaging findings suggested a subdural neoplastic lesion. A partial resection was performed for the lesion. Based on histopathological and immunohistochemical examinations, the patient was diagnosed with EMZMBCL. Whole-brain and intensity-modulated radiation therapies were administered as adjuvant therapies. The patient was discharged without neurological deficits.
Conclusion: EMZMBCL is a rare disease that should be considered in the differential diagnosis of subdural lesions, especially when there is no history of trauma or abnormalities in the coagulation system. The patient had a favorable outcome after selecting radiotherapy as the adjuvant therapy.
Keywords: Intracranial tumor, Meningioma, Primary extranodal marginal zone mucosa-associated lymphoid tissue-type B-cell lymphoma, Radiotherapy, Subdural hematoma
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) accounts for 2–4% of all central nervous system (CNS) tumors and is restricted to the CNS without other lesions.[
CASE DESCRIPTION
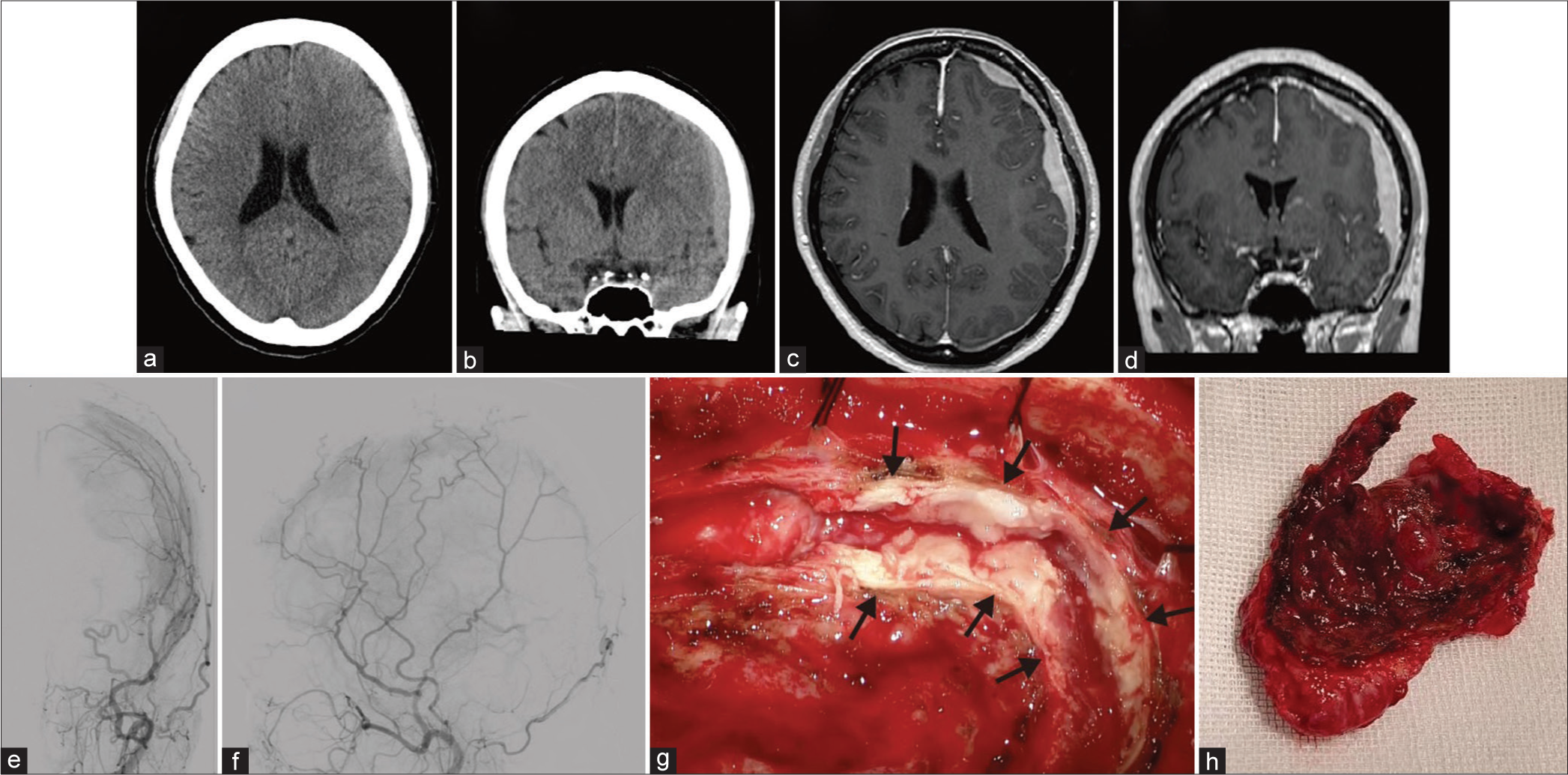
A 49-year-old woman with no significant medical history presented to our hospital with transient paresis in the right upper limb, dysarthria for 10 min, and ongoing numbness in the right upper limb. Although she presented with numbness in the right upper limb, a neurological examination revealed no deficits in the emergency room. Computed tomography (CT) of the head revealed multiple high-density lesions in the left frontal and temporoparietal regions [
Figure 1:
Axial (a) and coronal (b) computed tomography (CT) without contrast demonstrates a high-density subdural lesion. Axial (c) and coronal (d) magnetic resonance imaging with gadolinium contrast show residual contrast-enhancing extra-axial masses in the left frontal, temporal, and parietal lesions. Anteroposterior (e) and lateral (f) view of the left external carotid artery angiogram indicate arterial phase blush within the left convexity lesion. (g and h) Intraoperative findings showed a thickened dura mater (arrows) and a large tumor.
Histopathological and immunohistochemical examination
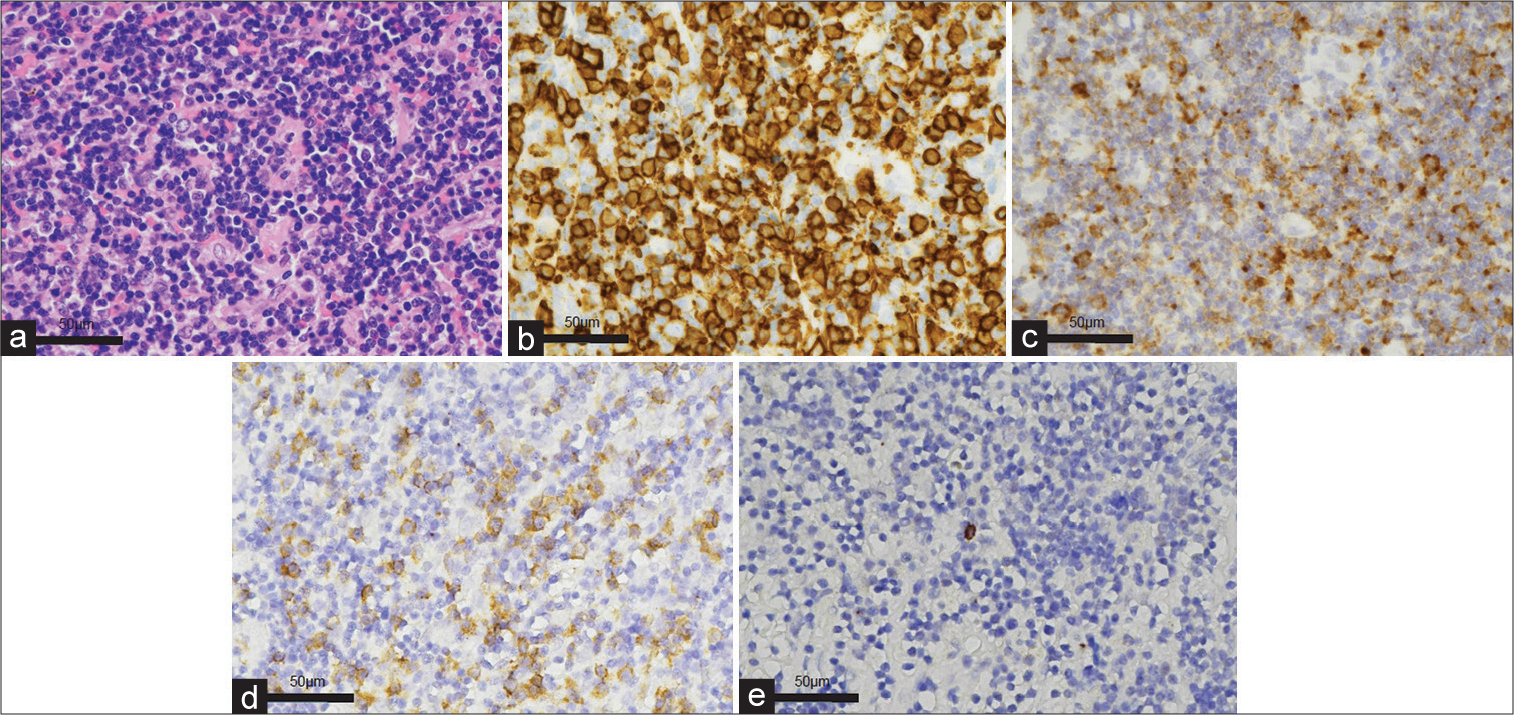
Histopathological examination revealed small lymphocytic infiltration [
Immunohistochemical examination revealed immunoglobulin light chain restriction (Kappa: Lambda = 100:2) [
Figure 2:
Histopathological and Immunohistochemical features of EMZMBCL. (a) Small lymphocytic infiltration (hematoxylin and eosin). (b) CD20 and (c) Insulin-like growth factor 2 mRNA-binding protein-3 (IMP3) immunohistochemical stains show diffuse positive. Immunohistochemical examination revealed immunoglobulin light-chain restriction (Kappa (d): Lambda (e) = 100:2). EMZMBCL: Extranodal marginal zone mucosa-associated lymphoid tissue-type B-cell lymphoma.
Radiotherapy
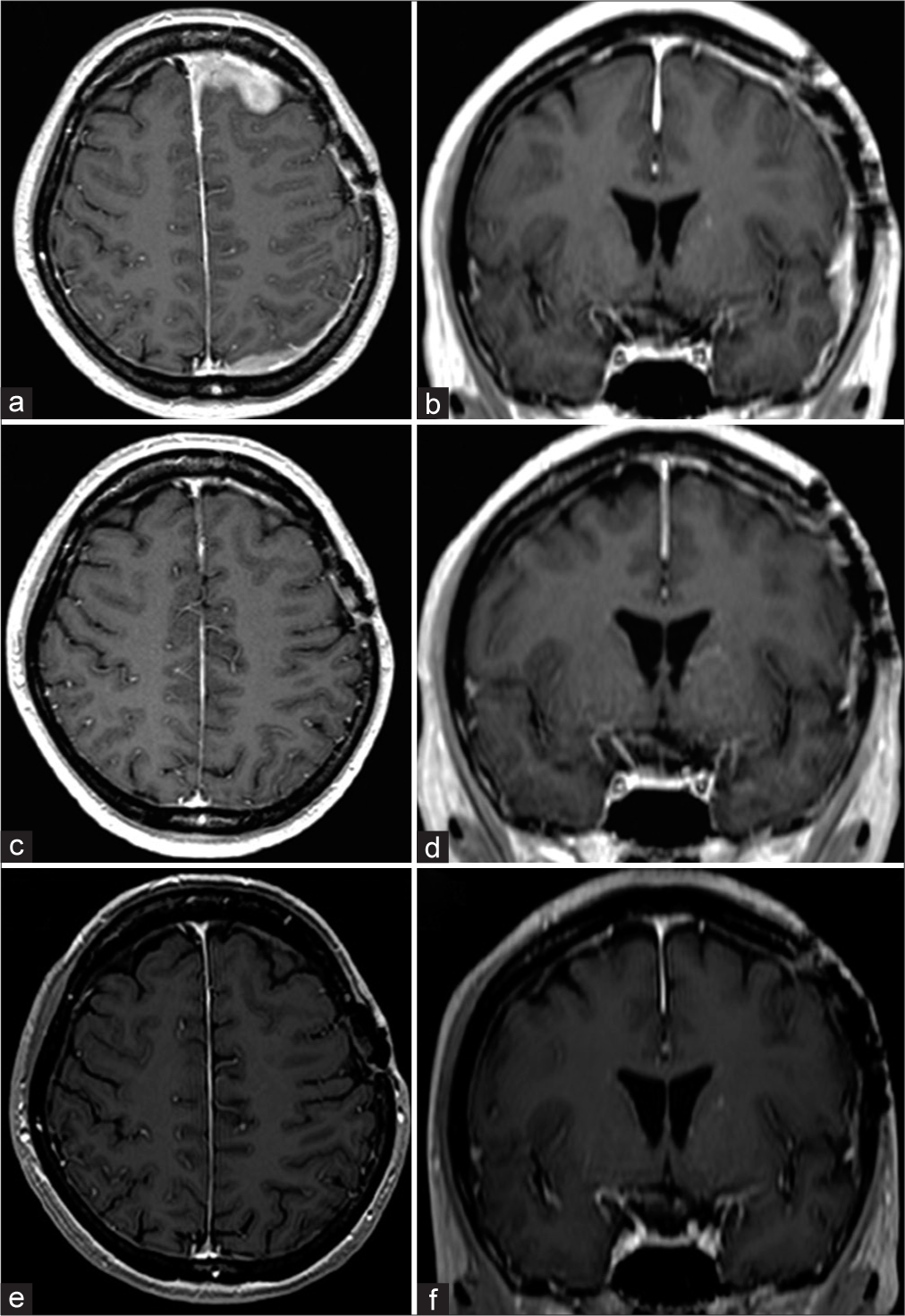
Whole-brain (21 Gy in 14 fractions) and intensity-modulated radiation therapy (9 Gy in 6 fractions) were administered as adjuvant therapy. The patient was discharged without neurological deficits. Compared with the pretherapy magnetic resonance imaging (MRI) [
Figure 3:
Preradiotherapy magnetic resonance imaging (MRI). Axial (a) and coronal (b) MRI with gadolinium contrast show residual contrast-enhancing extra-axial masses in the left frontal, temporal, and parietal lesions. The thickening and enhancement of the dura extend along the falx and the left frontal and parietal lobes. MRI at three months after radiation therapy. Axial (c) and coronal (d) MRI with gadolinium contrast indicate the disappearance of residual contrast-enhancing extra-axial masses in the left frontal, temporal, and parietal lesions and normalization of the dural thickening and enhancement. MRI at eight months after radiation therapy. Axial (e) and coronal (f) MRI with gadolinium contrast reveal no recurrence.
DISCUSSION
PCNSLs, which are extranodal NHLs, are most often DLBCLs and represent approximately 2–4% of intracranial neoplasms, which are high-grade tumors with poor outcomes.[
La Rocca et al. and Bustoros et al. have reported 126 and 104 cases of EMZMBCL, respectively.[
MALT lymphoma shows expression of pan-B cell markers (CD19, CD20, and CD79a) but does not express CD5 and CD10.[
Radiotherapy significantly prolongs overall survival in both gastric and nongastric extranodal marginal zone lymphomas.[
CONCLUSION
Here, we reported the case of a patient with EMZMBCL who initially presented with radiographic findings suggestive of a subdural hematoma. EMZMBCL is a rare disease that should be considered in the differential diagnosis of subdural lesions, especially when there is no history of trauma or coagulation system abnormalities. In this case report, the patient had a favorable outcome following partial tumor resection and adjuvant radiotherapy.
Ethical approval
This study was approval by the hospital ethics committee (No. 807, August 16, 2023).
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ariani R, Ballas L. Primary CNS extranodal marginal zone B-cell lymphoma: A case series of 2 patients treated with external beam radiation therapy. Case Rep Oncol. 2021. 14: 725-32
2. Batchelor TT. Primary central nervous system lymphoma: A curable disease. Hematol Oncol. 2019. 37: 15-8
3. Bustoros M, Liechty B, Zagzag D, Liu C, Shepherd T, Gruber D. A rare case of composite Dural extranodal marginal zone lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma. Front Neurol. 2018. 9: 267
4. Ferreri AJ, Marturano E. Primary CNS lymphoma. Best Pract Res Clin Haematol. 2012. 25: 119-30
5. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: Characteristic findings on traditional and advanced imaging. Am J Neuroradiol. 2011. 32: 984-92
6. Kim MY, Kim SM, Chung SY, Park MS. Dural marginal zone lymphoma confused with meningioma en plaque. J Korean Neurosurg Soc. 2007. 42: 220-3
7. La Rocca G, Auricchio AM, Mazzucchi E, Ius T, Della Pepa GM, Altieri R. Intracranial Dural based marginal zone MALT-type B-cell lymphoma: A case-based update and literature review. Br J Neurosurg. 2021. 26: 1-7
8. Lopetegui-Lia N, Delasos L, Asad SD, Kumar M, Harrison JS. Primary central nervous system marginal zone B-cell lymphoma arising from the Dural meninges: A case report and review of literature. Clin Case Rep. 2020. 8: 491-7
9. Park I, Huh J, Kim JH, Lee SW, Ryu MH, Kang YK. Primary central nervous system marginal zone B-cell lymphoma of the basal ganglia mimicking low-grade glioma: A case report and review of the literature. Clin Lymphoma Myeloma. 2008. 8: 305-8
10. Takeuchi I, Tanei T, Kuwabara K, Kato T, Naito T, Koketsu Y. Primary Dural mucosa-associated lymphoid tissue lymphoma mimicking falx meningioma: A case report. NMC Case Rep J. 2022. 9: 123-8
11. Tsutsumi Y, Ito S, Nagai J, Tateno T, Teshima T. Patients with marginal zone Dural lymphoma successfully treated with rituximab and bendamustine: A report of two cases. Mol Clin Oncol. 2021. 15: 208
12. Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O’Neill BP. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma : A primary lowgrade CNS lymphoma that mimics meningioma. J Clin Oncol. 2005. 23: 5718-27
13. Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: Biological and therapeutic relevance. Blood. 2016. 127: 2082-92