- Department of Neurosurgery, Apollo Health City, Filmnagar, Jubilee Hills, Hyderabad, Telangana, India,
- Department of Pathology, Apollo Health City, Filmnagar, Jubilee Hills, Hyderabad, Telangana, India.
Correspondence Address:
Amitava Ray, Department of Neurosurgery, Apollo Health City, Filmnagar, Jubilee Hills, Hyderabad, Telangana, India.
DOI:10.25259/SNI_665_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tarang K. Vora1, Rahul Lath1, Meenakshi Swain2, Amitava Ray1. Primary intracranial synovial sarcoma: A case report and review of literature. 30-Sep-2022;13:447
How to cite this URL: Tarang K. Vora1, Rahul Lath1, Meenakshi Swain2, Amitava Ray1. Primary intracranial synovial sarcoma: A case report and review of literature. 30-Sep-2022;13:447. Available from: https://surgicalneurologyint.com/surgicalint-articles/11903/
Abstract
Background: Primary intracranial synovial sarcomas (PrISS) are unusual dural based mesenchymal tumors seen most commonly in the supratentorial compartment. They can mimic a spontaneous intracranial hemorrhage or a high-grade glioma on imaging.
Case Description: A 31-year-old male presented with headache and right hemiparesis for 2 weeks. CT brain revealed a left frontal spontaneous intracerebral hemorrhage. PrISS revealed a heterogeneously ring enhancing solid cystic lesion with attachment to convexity dura. Intraoperatively, it mimicked a high-grade glioma. Histopathology report showed features of a synovial sarcoma, which was later confirmed with IHC. Classical SYT-SSX2 translocation was confirmed only on RTPCR after fluorescent in situ hybridization (FISH) was negative for same. Whole body positron emission tomography (PET-CT) did not show any extracranial tumor. Despite radiotherapy, there were recurrence and tumor progression at 6 months and the patient succumbed 11 months later.
Conclusion: PrISS is an unusual aggressive intracranial neoplasm that carries a worse prognosis when compared nonintracranial synovial sarcomas. Molecular cytogenetics (FISH and RTPCR) are essential for confirming the diagnosis, though FISH seems to have a lower sensitivity and can yield false negative results as was noted in this case.
Keywords: Fluorescent in situ hybridization, Primary intracranial synovial sarcoma, PrISS, RT-PCR, SYT-SSX2
INTRODUCTION
Primary intracranial synovial sarcoma (PrISS) is an unusual mesenchymal tumor within the intracranial space. Synovial sarcoma is an aggressive soft-tissue sarcoma that is generally seen in middle-aged patients, around the knee area, with a slight male predominance.[
CASE REPORT
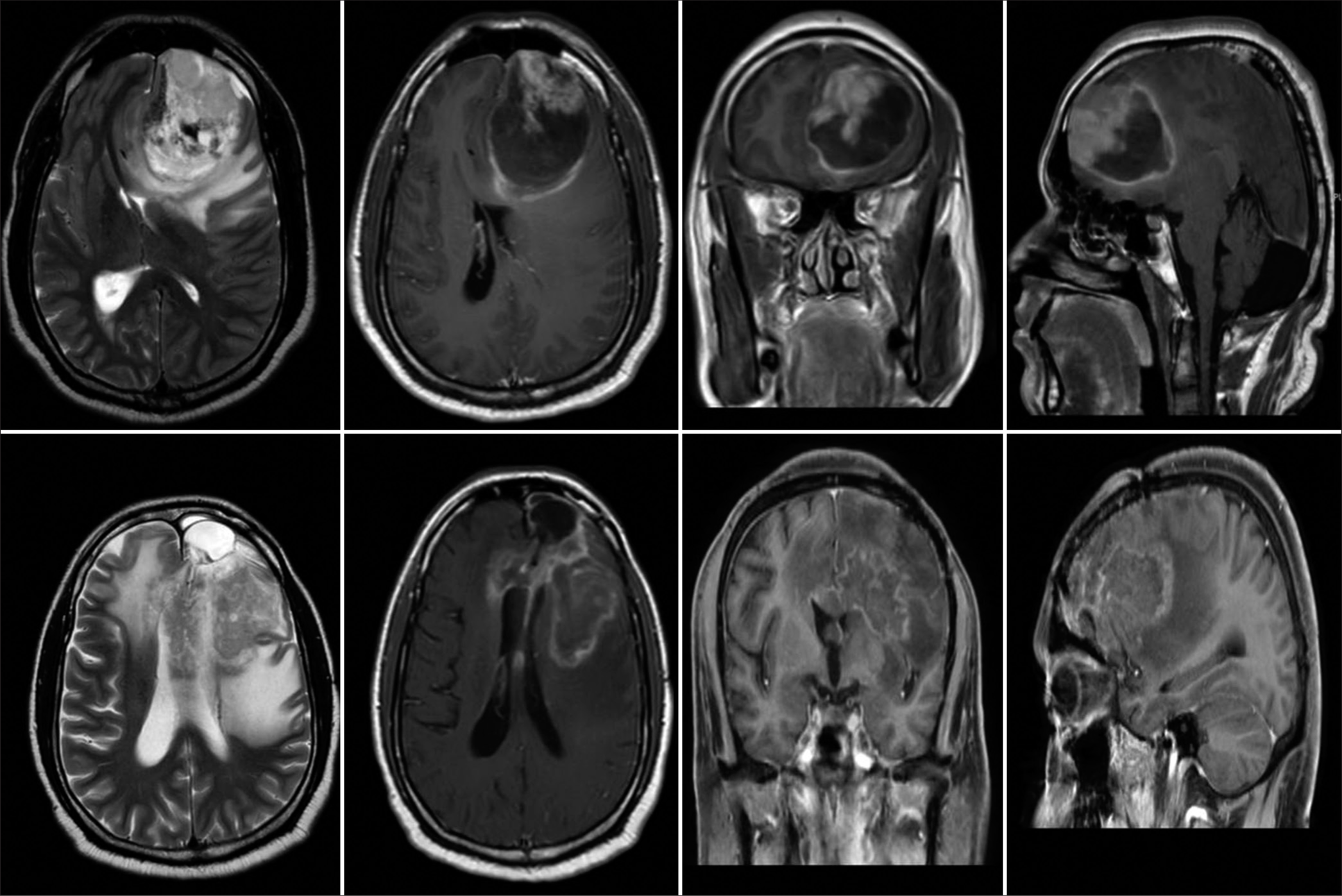
A 31-year-old male presented to us with severe persistent headache, right-sided weakness, and difficulty in speaking for 2 weeks at the height of the COVID pandemic. His admission GCS was E3V4M6 with Grade 4 power in the right upper and lower limbs. MRI brain showed the left frontal intra-axial heterogeneously enhancing tumor with an area of hemorrhage. Significant mass effect, perilesional edema, and mid line shift were also noticed [
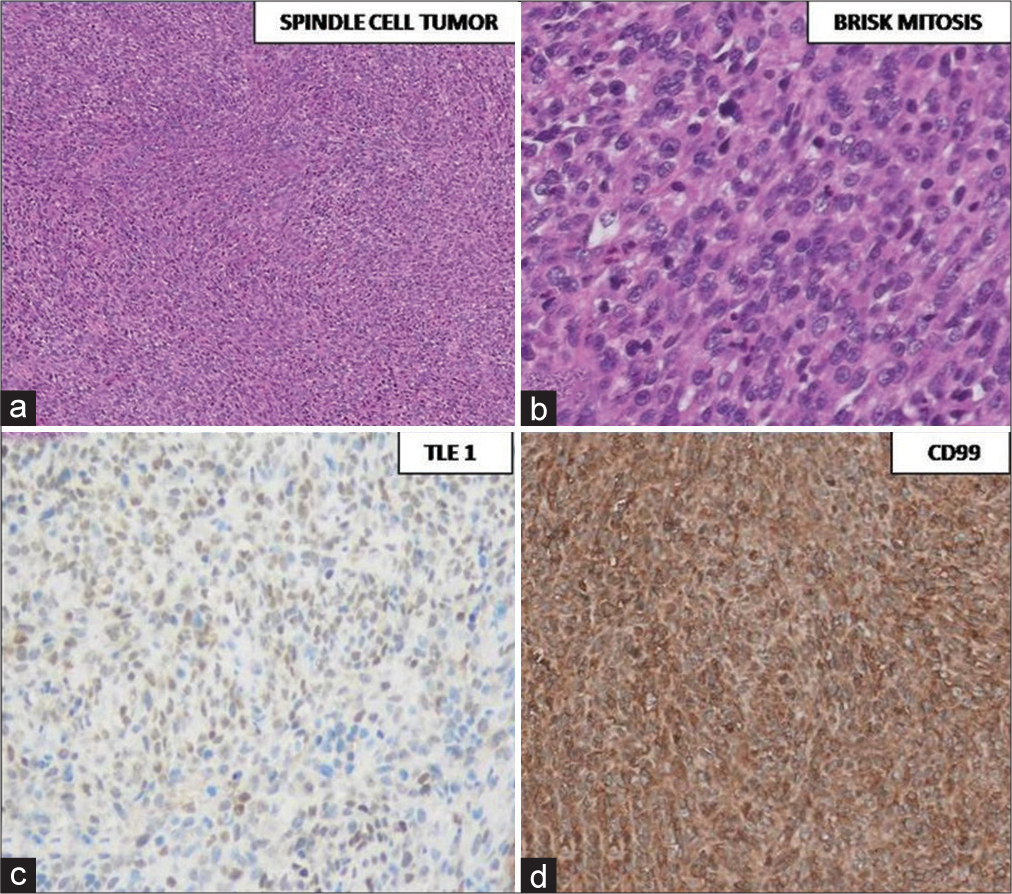
Histopathological examination [
Whole body PET CT was done to exclude a metastasis confirming the diagnosis as a PrISS. Postoperative imaging revealed a small residual disease. He received radiotherapy for the same (60 Gray in 30 fractions). At 6 months of follow-up, he was noticed to have tumor progression in spite of radiotherapy. [
DISCUSSION
Clinical symptoms and signs of PrISS depend on intracranial location of the tumor and may include dysphagia, pain, hoarseness, headache, or at times a palpable mass. The known sites of intracranial involvement are sellar region, skull base, cerebellum, and brain parenchyma. The preoperative diagnosis of PrISS is difficult and it may be thought to be an atypical meningioma, hemangiopericytoma, or a high-grade glioma. Synovial sarcoma may sometimes be confused with malignant meningioma because of their similar histological and immunohistochemical appearance (vimentinþ, EMAþ, and cytokeratinþ).[
Dural attachment and tumor origins
PrISS is an aggressive tumor associated with rapid neurological deterioration. Although “synovial” sarcoma has a predilection for particular areas, it is not actually associated with any synovial structure. Despite its name (a misnomer), it is no longer considered to be histologically derived from the synovium but rather from primitive mesenchymal cells.[
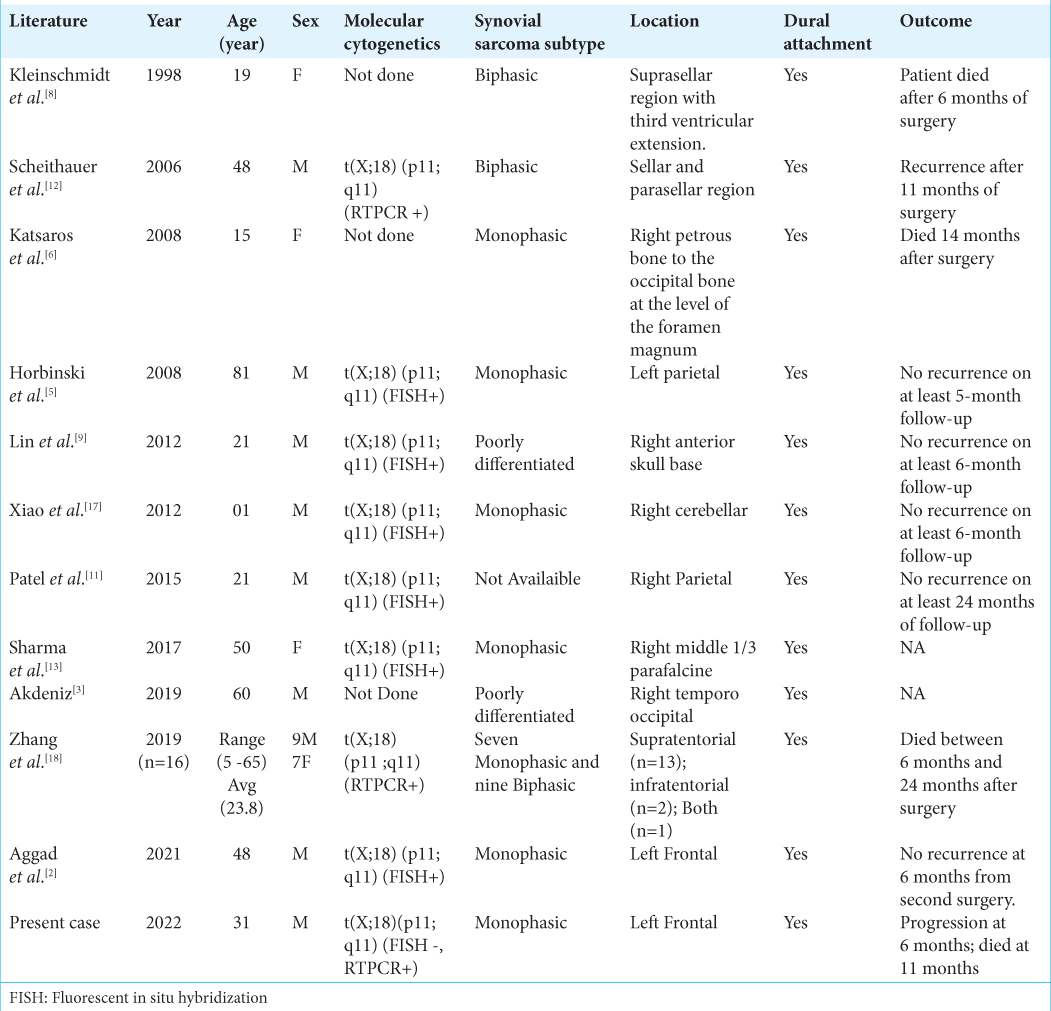
Review of the literature [ Table 1 ]
To the best of our knowledge, till date, 27 patients have been reported to have PrISS in English literature. [
Molecular cytogenetics: FISH versus RTPCR
Heuvel et al. compared the diagnostic accuracy of RT-PCR and FISH for synovial sarcomas. They verified SYT-SSX1/ SSX2 gene fusions and FISH analysis for SYT gene breaks on 50 specimens of formalin-fixed and paraffin embedded synovial sarcomas.[
Intratumoral hemorrhage in PrISS
PrISS has been reported to be a mimicker of spontaneous intracranial hemorrhage.[
Treatment and outcome
The treatment for systemic synovial sarcoma is surgical resection with wide margins followed by radiotherapy. Wide margins are difficult to achieve in PrISS in comparison to systemic synovial sarcomas. Standard treatment for PrISS is not yet optimized though, adjuvant therapies including radiation have demonstrated benefits for local recurrence and prognosis.[
CONCLUSION
PrISSs are unusual aggressive dural-based mesenchymal tumors with poor prognosis. They are seen most commonly in supratentorial compartment in 3rd or 4th decade of life. Radiological appearance of a supratentorial solid cystic lesion with dural attachment and intratumoral hemorrhage should raise suspicion for PrISS. Molecular cytogenetics including FISH and RTPCR are required for confirming the diagnosis, though FISH seems to have lower sensitivity than RTPCR and can yield false negative results.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adeeb N, Mortazavi MM, Tubbs RS, Cohen-Gadol AA. The cranial dura mater: A review of its history, embryology, and anatomy. Childs Nerv Syst. 2012. 28: 827-37
2. Aggad M, Gkasdaris G, Rousselot C, Destrieux C, François P, Velut S. Intracranial primary synovial sarcoma mimicking a spontaneous cerebral hematoma-a case report and review of the literature. Neurochirurgie. 2022. 68: 443-6
3. Akdeniz N. Rare occurrence of synovial sarcoma originating from dura mater. Turk J Oncol. 2019. 34: 52-5
4. Flannery T, Kano H, Niranjan A, Monaco EA, Flickinger JC, Kofler J. Gamma knife radiosurgery as a therapeutic strategy for intracranial sarcomatous metastases. Int J Radiat Oncol Biol Phys. 2010. 76: 513-9
5. Horbinski C, Cieply K, Bejjani GK, McFadden K. Primary intracranial dural-based synovial sarcoma with an unusual SYT fluorescence in situ hybridization pattern: Case report. J Neurosurg. 2008. 109: 897-903
6. Katsaros VK, Katsarou AA, Papadopoulou A, Floros D, Marangos N. Intracranial primary synovial sarcoma: Radiologicpathologic correlation. Neuroradiol J. 2008. 21: 362-7
7. Kawai A, Woodruff J, Healey JH, Brennan MF, Antonescu CR, Ladanyi M. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998. 338: 153-60
8. Kleinschmidt-Demasters BK, Mierau GW, Sze CI, Breeze RE, Greffe B, Lillehei KO. Unusual dural and skull-based mesenchymal neoplasms: A report of four cases. Hum Pathol. 1998. 29: 240-5
9. Lin YJ, Yang Q, Tian X, Li B, Li Z. Unusual primary intracranial dural-based poorly differentiated synovial sarcoma with t(X; 18)(p11; q11). Neuropathology. 2013. 33: 75-82
10. Miettinen M, Virtanen I. Synovial sarcoma--a misnomer. Am J Pathol. 1984. 117: 18-25
11. Patel M, Li L, Nguyen HS, Doan N, Sinson G, Mueller W. Primary intracranial synovial sarcoma. Case Rep Neurol Med. 2016. 2016: e5608315
12. Scheithauer BW, Silva AI, Kattner K, Seibly J, Oliveira AM, Kovacs K. Synovial sarcoma of the sellar region. Neuro Oncol. 2007. 9: 454-9
13. Sharma S, Sharma A, Lobo G, Nayak M, Pradhan D, Samriti . Primary dura-based synovial sarcoma of the parafalcine region of brain. Pathol Res Pract. 2017. 213: 868-71
14. Stacchiotti S, van Tine BA. Synovial sarcoma: Current concepts and future perspectives. J Clin Oncol. 2018. 36: 180-7
15. Takeyama R, Fukuda K, Kouzaki Y, Koga T, Hayashi S, Ohtani H. Intracerebral hemorrhage due to vasculitis following COVID-19 vaccination: A case report. Acta Neurochir (Wien). 2022. 164: 543-7
16. Ten Heuvel SE, Hoekstra HJ, Suurmeijer AJ. Diagnostic accuracy of FISH and RT-PCR in 50 routinely processed synovial sarcomas. Appl Immunohistochem Mol Morphol. 2008. 16: 246-50
17. Xiao GY, Pan BC, Tian XY, Li Y, Li B, Li Z. Synovial sarcoma in cerebellum: A case report and literature review. Brain Tumor Pathol. 2014. 31: 68-75
18. Zhang G, Xiao B, Huang H, Zhang Y, Zhang X, Zhang J. Intracranial synovial sarcoma: A clinical, radiological and pathological study of 16 cases. Eur J Surg Oncol. 2019. 45: 2379-85